��Ŀ����
11���ס�����ͬѧ�ⶨ��pH��ֽ��֤���������ᣬ�����ֱ��ǣ��ף��ٳ�ȡһ�����ı�����ȷ����0.1mol/L�Ĵ�����Һ100mL
����pH��ֽ�������Һ��pH������֤�����������ᣮ
�ң��ٳ�ȡһ�����ı�����ȷ����pH=1�Ĵ�����Һ100mL��
��ȡ������Һ10mL����ˮϡ��Ϊ100mL��
����pH��ֽ�������Һ��pH������֤�����������ᣮ
��1�����������ĵڢٲ��У���Ҫ�õ��Ķ���������100mL����ƿ��������ƽ��
��2����Ҫ˵��pH��ֽ��ʹ�÷�����ȡһС��pH��ֽ���ڲ���Ƭ�ϣ��ò�����պȡ����Һ����pH��ֽ�ϣ����ձ�ɫ����������Һ��pH��
��3�������У�˵������������������Dz�ô�����Һ��pH��1��ѡ�������������=�������ҷ����У�˵������������������Dz�ô�����Һ��pH��2��ѡ�������������=������
��4������Ҳ���һ����pH��ֽ��֤������������ĺ����������еķ��������Բ�������pH��ֽ��ʹ�÷�������������Ӧ����ʵ�鷽��������ͽ��ۣ����ƴ�������Һ����pH��ֽ����Һ��pH�������ҺpH����7������֤�����������ᣮ
���� ��1������һ�����ʵ���Ũ�ȵ���Һ��Ҫ�õ�����ƿ�������������õ�������ƽ��
��2������pH��ֽ����ȷʹ�÷������н��
��3��������ˮ��Һ�ﲿ�ֵ��룬����������Ũ��С����Ũ�ȣ������ˮϡ��10��PH�仯����һ����λ��
��4���������ô�������Һˮ����ʾ�����жϴ���Ϊ������ʣ�
��� �⣺��1������100mL��Һ��Ҫ�õ�100mL������ƿ����ȡ��������Ҫ�õ�������ƽ���������������ĵڢٲ��У���Ҫ�õ��Ķ��������ǣ�100mL����ƿ��������ƽ
�ʴ�Ϊ��100mL����ƿ��������ƽ��
��2��pH��ֽ��ʹ�÷���Ϊ��ȡһС��pH��ֽ���ڲ���Ƭ�ϣ��ò�����պȡ����Һ����pH��ֽ�ϣ����ձ�ɫ����������Һ��pH��
�ʴ�Ϊ��ȡһС��pH��ֽ���ڲ���Ƭ�ϣ��ò�����պȡ����Һ����pH��ֽ�ϣ����ձ�ɫ����������Һ��pH��
��3��������ˮ��Һ�ﲿ�ֵ��룬����������Ũ��С����Ũ�ȣ����pH��1�������ˮϡ��10��pH�仯����һ����λ������1��pH��2���ʴ�Ϊ����������
��4���������ô�������Һˮ��ԭ����Ʒ�����ǿ����������Һˮ��ʼ��ԣ�˵��������ӶԵĴ���Ϊ���ᣬ��������Ϊ�����ƴ�������Һ����pH��ֽ����Һ��pH�������ҺpH����7������֤�����������ᣬ
�ʴ�Ϊ�����ƴ�������Һ����pH��ֽ����Һ��pH�������ҺpH����7������֤�����������ᣮ
���� ���⿼���˱Ƚ��������ǿ����������Ŀ�Ѷ��еȣ���ȷ��ѧʵ�������������Ϊ���ؼ���ע������������ʵĸ���жϷ���������������ѧ���Ļ�ѧʵ��������

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д� ������˵����ȷ���ǣ�������
������˵����ȷ���ǣ�������| A�� | ���� �Ľṹ�ص��֪������ϩ�ķ���ʽΪC10H8 �Ľṹ�ص��֪������ϩ�ķ���ʽΪC10H8 | |
| B�� | ���³�ѹ�»�����ϩ��һ��������ˮ����ɫ���� | |
| C�� | ������ϩ����ʹ��ˮ��ɫ������ʹ����KMnO4��Һ��ɫ | |
| D�� | ��ϩ�ͻ�����ϩ��Ϊͬϵ�� |
| A�� | Na2CO3���ȶ��Խ���Na2CO3•10H2O��NaHCO3֮�� | |
| B�� | �������Ƽ���Ƶõļ���ָNaHCO3 | |
| C�� | �����������Na2CO3 ��NaHCO3���� | |
| D�� | ������ʱ�ɴ����ռ�ʹ�ã������ܽ�ʯӢ������������ |
��ˮ�������ģ�ͣ�

����觵���Ҫ�ɷ�Ϊ������
��
 ��������3-��-1-����
��������3-��-1-�����ܾ���ϩ����ṹ��ʽΪCH2=CH2
��HClO�Ľṹ��ʽΪ��H-Cl-O
��16O2��18O2��Ϊͬλ�أ�
| A�� | �٢ۢ� | B�� | �٢ݢ� | C�� | �ۢ� | D�� | �ܢ� |
| A�� | ������Һ��c��Ag+��•c��Cl-����Ksp��AgCl�� | |
| B�� | ����ʵ��˵��Cl-��NH4+���к�ǿ�Ľ���� | |
| C�� | ������Һ���γ����ѵ�������� | |
| D�� | ����������NH3•H2O�ĵ��볣������ |
����Ʒ�����ɫ�⣻
���⾵�ֹ⣻
�ۼ������������⣻
��ʯ������
�����۹⣻
��ȼ�ŵ������ҹ���г�������ͷף�
���չ��ͨ�緢�⣻
����ȴ�ᾧ��
����ɫ��Ӧ��
| A�� | �ڢܢݢ�� | B�� | �٢ۢޢߢ� | C�� | �٢ۢݢޢ� | D�� | �٢ڢۢݢޢ� |

 ��
��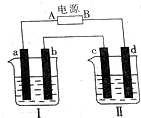 ��ͼ��ʾװ���У��ձ����зֱ�ʢ��200g 9.4%��Cu��NO3��2��Һ�������ı���K2SO3��Һ�����õ缫��Ϊ���Ե缫����ش��������⣺
��ͼ��ʾװ���У��ձ����зֱ�ʢ��200g 9.4%��Cu��NO3��2��Һ�������ı���K2SO3��Һ�����õ缫��Ϊ���Ե缫����ش��������⣺ ±�صĵ��ʼ��仯������������������Ӧ�ù㷺��
±�صĵ��ʼ��仯������������������Ӧ�ù㷺�� ����${\;}_{1}^{2}$H����
����${\;}_{1}^{2}$H����