��Ŀ����
����Ŀ���о����ʵĺϳɻ��Ʊ����л���ѧ������ѧ����Ҫ����֮һ��
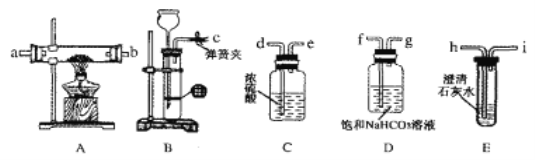
(1)ijʵ��С��̽��ʵ�����Ʊ���ˮ�Ȼ�þ�ķ��������������ͼװ�á�

�ٷ�Һ©���е�A������___________ (���Լ�����)��
��������ѧ������������ijͬѧ�ڿհ����ڲ�������ʵ��װ�ã���ѡ����Լ���____ (�����)
A��ϡNaOH��Һ B����ˮ�Ȼ��� C��ϡ���� D��Ũ����
(2)ʵ�����Ʊ����ռ�������ϩ��
���е�ͬѧͨ���Ҵ��Ʊ���ϩ��д����ػ�ѧ����ʽ��_______________����ʵ����Ҵ��⣬������Լ�����Ʒ(����������)��_______________________��
���е�ͬѧ̽�������Ʊ���ϩ�ķ��������������ͼװ���Ʊ���ϩ��ʵ��������Ͳ�ڱڸ��Ž϶���ɫ��״Һ�壬�ҵõ������������塣�����������ʺܵ͵�ԭ����Ҫ��_______��

���𰸡�Ũ���� B(��D)��A CH3CH2OH![]() CH2=CH2����H2O Ũ���ᡢ��ʯ������������Һ �����¶ȹ��ߣ�����������ӷ�(�������鷢���˸���Ӧ)
CH2=CH2����H2O Ũ���ᡢ��ʯ������������Һ �����¶ȹ��ߣ�����������ӷ�(�������鷢���˸���Ӧ)
��������
(1)����Ũ�������ˮ�ԡ��ѻӷ��ԣ�ʹŨ����ӷ�����HCl���壻HCl�Ǵ�����Ⱦ�Ҫ��ˮ��NaOH��Һ����β����������β������װ�������װ��֮����Ҫ����ʢ���Ȼ��Ƶĸ����(��ʢ��Ũ�����ϴ��ƿ)��
(2)���Ҵ���Ũ���������¼���170�淢����ȥ��Ӧ������ϩ����Ӧ�лᷢ������Ӧ���ж�����̼�������������ɣ�������������Һ���ճ�ȥ������̼����������Һ�������Ҫ��ֹ���У�����Ҫ�����ʯ��
�ڷ��������������鷢����ȥ��Ӧ������ϩ������ͼ��װ���Ʊ���ϩ��ʵ��������Ͳ�ڱڸ��Ž϶���ɫ��״Һ�壬�ҵõ������������壬˵��������ϩ���٣�������������ӷ������Կ���������������װ�ã�������������������������������ˮԡ���ȿ��Ʒ�Ӧ�¶ȣ�����������ӷ�����Ӧ������HBr��HBr��������ˮ��������ˮ���ռ���ϩ���ᷢ��������
(1)Ũ���������ˮ�Ժ��ѻӷ��ԣ��������Ũ�����У�Ũ��������Ũ�����е�ˮ�ų�������ʹHCl����ӷ����Ӷ�������ȡHCl���壻HCl�Ǵ�����Ⱦ��ھ���Ũ����������뷴Ӧװ�ã�ʹ�Ȼ�þ������HCl�����м��ȿ��Եõ���ˮ�Ȼ�þ��Ϊ��ֹ�õ�����ˮ�Ȼ�þ���⣬��ͨ��һ������ˮ�ֵĸ���װ��ѡ��B��D��Ȼ���ݳ���HCl������NaOH��Һ���գ���ֹ����Ⱦ���������ѡ����B(��D)��A��
(2)���Ҵ���Ũ�����ڼ���170��ʱ������ȥ��Ӧ������ϩ��ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH3CH2OH![]() CH2=CH2����H2O��Һ̬�������ȣ�Ϊ��ֹ���У�Ҫ�������Ƭ���ʯ������Ũ�������ǿ�����ԣ��Ҵ����л�ԭ�ԣ����ᷢ������Ӧ����CO2��SO2���ɣ�Ϊ��ֹ��Ⱦ������Ҫ������������Һ���ճ�ȥβ��������������Լ�����Ʒ(����������)���Ҵ��⣬���У�Ũ���ᡢ����������Һ����ʯ��
CH2=CH2����H2O��Һ̬�������ȣ�Ϊ��ֹ���У�Ҫ�������Ƭ���ʯ������Ũ�������ǿ�����ԣ��Ҵ����л�ԭ�ԣ����ᷢ������Ӧ����CO2��SO2���ɣ�Ϊ��ֹ��Ⱦ������Ҫ������������Һ���ճ�ȥβ��������������Լ�����Ʒ(����������)���Ҵ��⣬���У�Ũ���ᡢ����������Һ����ʯ��
�ڷ��������������鷢����ȥ��Ӧ������ϩ������ͼ��װ���Ʊ���ϩ��ʵ��������Ͳ�ڱڸ��Ž϶���ɫ��״Һ�壬�ҵõ������������壬˵��������ϩ���١�������ʺܵ͵�ԭ����Ҫ�ǣ������¶ȹ��ߣ�����������δ��Ӧ�ʹ����ӷ���Ҳ�����������鷢������Ӧ�ȡ�

 ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�����Ŀ��þ�Ͻ�þ�Ļ����������������������Ź㷺��Ӧ�á�
(1)þ��Ԫ�����ڱ��е�λ����__________________��
(2)��ˮ��þʯ(��Ҫ�ɷ�ΪMgCl2��6H2O)�Ʊ�����þ�Ĺؼ��������£�

�ù����п�ѭ��ʹ�õ�������______________��
(3)�������Mg(AlH4)2��110��200 ��ķ�ӦΪMg(AlH4)2=MgH2��2Al��3H2����ÿת��6 mol�����������������ʵ���Ϊ________mol��
(4)��ʽ̼��þ�ܶ�С��������Ʒ���������ϣ����ø���MgCO3��(NH4)2CO3��2H2O��ԭ���Ʊ����Ʊ������У���Ҫ�õ�±ˮ(�Ȼ�þ��Һ)��ij����С���ó����ζ���������Ʒ��Cl���ĺ�������ȡ6.100 0 g��Ʒ�����������ܽ⣬��ϡ�͵Ȳ����������500 mL����Һ��
a��ȷ��ȡ25.00 mL ����Һ����0.100 0 mol/L AgNO3��Һ�ζ����ζ�ǰ��ζ����е�Һ�������ͼ��ʾ����ζ����������ı�Һ�����Ϊ________mL��

b.
AgCl | AgBr | AgI | Ag2CrO4 | |
Ksp | 2��10��10 | 5.4��10��13 | 8.3��10��17 | 2��10��12 |
��ɫ | �� | ���� | �� | ש�� |
�����ϱ����ݼ���Ϣ�������ζ�ʱ������ָʾ������________(���������)��
��CaCl2������NaBr������NaI������K2CrO4
����Ŀ���������أ�![]() ���ܴ�ʹ����������ȷ������ӷ������������ʵ���Ʊ��������ز��ⶨ�䴿�ȣ�
���ܴ�ʹ����������ȷ������ӷ������������ʵ���Ʊ��������ز��ⶨ�䴿�ȣ�
I.�Ʊ�
����1���Ʊ������ᶡ����![]() ��
��
![]()
��Ӧװ����ͼ1���г�װ����ȥ�������ձ������μ���ϡ���ᡢ����������������Һ������Ӧ��ȫ������ϲ���״���![]() ��
��![]() �Ļ����Һϴ�����Σ���������á�
�Ļ����Һϴ�����Σ���������á�
����2���Ʊ���������
![]()
��Ӧװ����ͼ2���гּ�����װ��·ȥ����������A�м���![]() �Ҵ���Һ��
�Ҵ���Һ��![]() ��������
��������![]() ���������ᶡ��������ԡ���ȣ���Ӧ��ȫ�������ؼ�������������ԡ��ȴ�����ˣ�������ˮ�Ҵ�ϴ�ӣ�������ˮ����ϴ�ӣ��ڿ�������
���������ᶡ��������ԡ���ȣ���Ӧ��ȫ�������ؼ�������������ԡ��ȴ�����ˣ�������ˮ�Ҵ�ϴ�ӣ�������ˮ����ϴ�ӣ��ڿ�������![]() ���
���


��������������£�
���� | ��ɫ��״̬ | �е㣨�棩 | �ܽ��� |
| ��ɫ���� | �����ֽ� | ������ˮ�������Ҵ������������� |
| ��ɫҺ�� | 118 | ����ˮ�����Ҵ������ѻ��� |
| ��ɫ��ɫ��״Һ�� | 78 | ������ˮ�����Ҵ������ѻ��� |
| ��ɫ��״Һ�� | 118 | ��ˮ���Ҵ����ܣ����������� |
��ش�
��1������A������Ϊ_____________.
��2������1�з���������ᶡ���IJ�������Ϊ_____________������1����NaCl��NaHCO3�Ļ����Һϴ�ӵ�Ŀ����__________________________.
��3������2�б�ԡ��ȴ��Ŀ����__________________________������2�и����Ʒ���¶ȿ�����55~60�棬ԭ����__________________________
��4��������߲�Ʒ�Ĵ��ȣ�����_____________�����ţ��н����ؽᾧ��
A.��ˮ�Ҵ� B.��ˮ���� C.ˮ D.�Ҵ���ˮ��Һ
��.�ֹ��ȷ��ⶨ��Ʒ�Ĵ���
ԭ����![]() ��
��![]() ��Ӧ�dz����������ɺ�ɫ������һ�������²�����ɫ��Һ������ȣ����á�
��Ӧ�dz����������ɺ�ɫ������һ�������²�����ɫ��Һ������ȣ����á�![]() ����ȡ�����ȷ����Ʒ��Һ�е�
����ȡ�����ȷ����Ʒ��Һ�е�![]() ���ⶨ�������£�
���ⶨ�������£�
����![]() Ʒ������
Ʒ������![]() ����Һ��
����Һ��
������һ����ͬ�����![]() ����ͬŨ�ȵ�
����ͬŨ�ȵ�![]() ����Һ���ֱ����
����Һ���ֱ����![]() ��������
��������![]() ����Һ��ҡ�ȣ���������ȣ����Ʊ���Һ��
����Һ��ҡ�ȣ���������ȣ����Ʊ���Һ��![]() ������ȵĹ�ϵ���ߣ���ͼ��
������ȵĹ�ϵ���ߣ���ͼ��
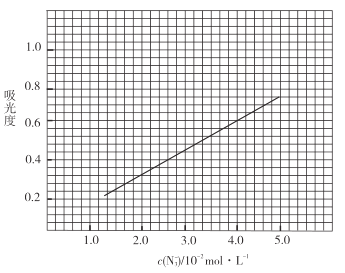
�۲�Ʒ�ⶨ����ȡ0.360g��Ʒ�����![]() ��Һ��ȡ��
��Һ��ȡ��![]() �ڱ����У�����
�ڱ����У�����![]() ��������
��������![]() ����Һ��ҡ�ȣ���������Ϊ0.6��
����Һ��ҡ�ȣ���������Ϊ0.6��
��5��ʵ������![]() ��������
��������![]() ����Һ�ķ���Ϊ_________________.
����Һ�ķ���Ϊ_________________.
��6����Ʒ�Ĵ���Ϊ_________________�������м����![]() ����Һ�����Խ���Ʒ��ȫ��Ӧ�����õIJ�Ʒ����________________���ƫ�ߡ���ƫ�͡�����Ӱ�족����
����Һ�����Խ���Ʒ��ȫ��Ӧ�����õIJ�Ʒ����________________���ƫ�ߡ���ƫ�͡�����Ӱ�족����