��Ŀ����
��֪HClO H+ +ClO-��Ϊ��̽��HClO��Ư���ԣ�ij��ѧ��ȤС���A��B��C��D��ѡ�������Ʊ���������������������������ʵ�顣
H+ +ClO-��Ϊ��̽��HClO��Ư���ԣ�ij��ѧ��ȤС���A��B��C��D��ѡ�������Ʊ���������������������������ʵ�顣
 H+ +ClO-��Ϊ��̽��HClO��Ư���ԣ�ij��ѧ��ȤС���A��B��C��D��ѡ�������Ʊ���������������������������ʵ�顣
H+ +ClO-��Ϊ��̽��HClO��Ư���ԣ�ij��ѧ��ȤС���A��B��C��D��ѡ�������Ʊ���������������������������ʵ�顣 
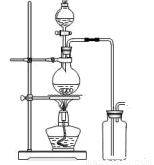
(1)�������������ң��������ռ����壬�Ʊ��������������ʱ����������˳��Ϊ___���� д��ĸ����
(2)�����������ͨ���E�ļ���ƿ��ʪ�����ɫ�����ܿ���ɫ�����������ɫ����û�кܿ��� ɫ���ɴ˼�ͬѧ��Ϊ�����������___����С���û�С�����ͬ��Ư���ԣ���ʪ������____ Ư���ԡ�
(3)��ͬѧ��Ϊ��ȤС��������ȱ�ݣ�Ӧ���������ģ�____��
(4)E���ձ���NaOH��Һ�����������___���ձ�����������Ӧ�Ļ�ѧ����ʽΪ____��
(5)Ϊ��֤ʵ�鰲ȫ����������ÿ����1.12 L����״��������������ͨ��ʱ����ʵ�����ʱ 5 min��С�ձ���ʢ2 mol��L-1 NaOH��Һ���������ӦΪ___mL��
(6)��ͬѧ����ȡ�ĸ�������������м�������ˮ�Ƶ���ˮ����������ˮ��������ʵ�飬ʵ��� �������������¡�
(2)�����������ͨ���E�ļ���ƿ��ʪ�����ɫ�����ܿ���ɫ�����������ɫ����û�кܿ��� ɫ���ɴ˼�ͬѧ��Ϊ�����������___����С���û�С�����ͬ��Ư���ԣ���ʪ������____ Ư���ԡ�
(3)��ͬѧ��Ϊ��ȤС��������ȱ�ݣ�Ӧ���������ģ�____��
(4)E���ձ���NaOH��Һ�����������___���ձ�����������Ӧ�Ļ�ѧ����ʽΪ____��
(5)Ϊ��֤ʵ�鰲ȫ����������ÿ����1.12 L����״��������������ͨ��ʱ����ʵ�����ʱ 5 min��С�ձ���ʢ2 mol��L-1 NaOH��Һ���������ӦΪ___mL��
(6)��ͬѧ����ȡ�ĸ�������������м�������ˮ�Ƶ���ˮ����������ˮ��������ʵ�飬ʵ��� �������������¡�

�����������Ƴ�����Ӧ�����Ƿ������___��������������˵�����ɣ�����������������д��:____ .
(1)A��C��C
(2)û�У���
(3)��E����������ƿ֮������һ������װ�û�E��ǰ��������ƿ����˳��
(4)���ն����Cl2��Cl2 +2NaOH=NaCl+ NaClO+ H2O
(5)250
(6)��������û������֤�������������Ư���ԣ�Ҳû����֤�����Ƿ����Ư����
(2)û�У���
(3)��E����������ƿ֮������һ������װ�û�E��ǰ��������ƿ����˳��
(4)���ն����Cl2��Cl2 +2NaOH=NaCl+ NaClO+ H2O
(5)250
(6)��������û������֤�������������Ư���ԣ�Ҳû����֤�����Ƿ����Ư����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ







 �ĵ���ʽ
�ĵ���ʽ
 H++HCO3-
Ka1 =4��45��10-7
H++HCO3-
Ka1 =4��45��10-7