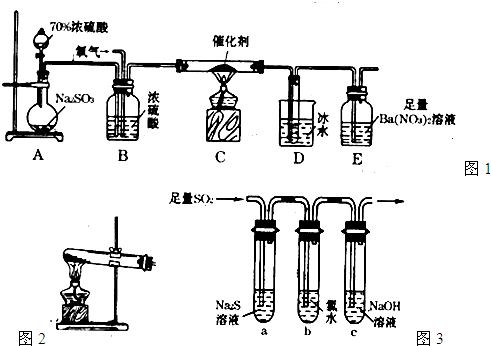
��Ŀ����
(8��)ʵ������ NaHSO4 ,Ba(OH)2, NH3 ? H2O NaHCO3��KAl(SO4)2������ɫ��Һ������ͨ������֮������Ӧ���������м��𡣲������ʼ�ķ�Ӧ�������±���
���С� ����ʾ�����������ʣ���
����ʾ�����������ʣ��� ����ʾ���ɳ�����
����ʾ���ɳ�����
����������Ϣ���ش��������⣺
(1)B��E�Ļ�ѧʽ�ֱ�Ϊ_______��___________��
(2) д��A�ĵ��뷽��ʽ___________________________����
(3) C��D����Һ��Ӧ�����ӷ���ʽΪ___________________________��
(4) ����0.1 mol���ʵ�D��Һ�еμ�E��Һ�������ɳ��������ʵ���֮�����Ϊ_________mol��
��8�֣���1��NaHSO4��Ba(OH)2 ��2�֣� ��2��NH3��H2O NH4++OH-��2�֣�
NH4++OH-��2�֣�
��3��Al3++3HCO3-= Al(OH)3��+3CO2����2�֣� ��4�� 0.25 ��2�֣�
����

��ϰ��ϵ�д�
 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д� ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
�����Ŀ