��Ŀ����
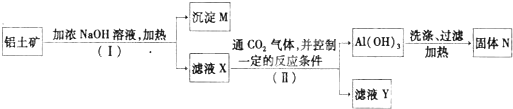
��ҵ������������Ҫ�ɷ���Al2O3������������Fe2O3��SiO2����ȡұ������ԭ����������������������ͼ��

��1��ԭ��A��������______������ٷ�Ӧ�����ӷ���ʽ��______��
��2����Һ1��Ҫ�����Թ���ԭ��B��ԭ��B�Ļ�ѧʽ��______������ڷ�Ӧ�����ӷ���ʽ�ǣ�______��
��3������۵Ļ�ѧ����ʽ�ǣ�______��
��4�����ʡȥ����٣����ܽ��������ǴӼ���ԭ��B��ʼ�������������䣬��������������ȡ��ʲôӰ��______��
�⣺�ɹ������̿�֪�����ͨ���Ϊ������̼�����ɵij���Ϊ����������������Һ�ں���AlO2-�������Ӧ�����������ƣ�������Һ���к���Al3+�������ӦΪ�������ᣬ��ȥSiO2��
��1��������������֪������ټ������ᣬ��ȥSiO2������ԭ��AΪ���ᣬ
���������ᷴӦ���ӷ���ʽΪAl2O3+6H+=2Al3++3H2O�������������ᷴӦ���ӷ���ʽΪFe2O3+6H+=2Fe3++3H2O��
�ʴ�Ϊ��Al2O3+6H+=2Al3++3H2O��Fe2O3+6H+=2Fe3++3H2O��
��2������ټ���������ᣬ��Һ���к���Al3+��Fe3+��H+�ȣ�Ӧ�����������ƣ���ȥ�����ӣ���������ת��ΪAlO2-����ӦΪ H++OH-=H2O��Fe3++3OH-=Fe��OH��3����Al3++4OH-=AlO2-+2H2O��
�ʴ�Ϊ��NaOH�� H++OH-=H2O��Fe3++3OH-=Fe��OH��3��Al3++4OH-=AlO2-+2H2O��
��3����Һ�ں���AlO2-�������ͨ�������̼����AlO2-��Ӧ���ɵij���Ϊ����������
��Ӧ��ѧ����ʽΪNaAlO2+CO2+2H2O=Al��OH��3��+NaHCO3��
�ʴ�Ϊ��NaAlO2+CO2+2H2O=Al��OH��3��+NaHCO3��
��4������NaOH��Һ�ܽ����������в���SiO2�ܽ���NaOH��Һ�����ɹ����ƣ�ͨ�������̼�����ɹ�����������ʹ�����Ƶõ�Al2O3����SiO2���ʣ�
�ʴ�Ϊ������NaOH��Һ�ܽ����������в���SiO2�ܽ���NaOH��Һ�����ɹ����ƣ����ʹ�����Ƶõ�Al2O3����SiO2���ʣ�
������SiO2�������Ӧ��Fe2O3���������Ʋ���Ӧ���������ܷ�Ӧ����ƫ��������ͨ������Ķ�����̼����������������������
�ɹ������̿�֪�����ͨ���Ϊ������̼�����ɵij���Ϊ����������������Һ�ں���AlO2-�������Ӧ�����������ƣ���ȥ�����ӣ���������ת��ΪAlO2-��������Һ���к���Al3+�������ӦΪ�������ᣬ��ȥSiO2��
�����������Կ�ͼ����ʽ�������Ļ���������ʣ��Ѷ��еȣ����ұ������������ԭ�����Ƕ�֪ʶǨ�Ƶ��ۺ����ã�
��1��������������֪������ټ������ᣬ��ȥSiO2������ԭ��AΪ���ᣬ
���������ᷴӦ���ӷ���ʽΪAl2O3+6H+=2Al3++3H2O�������������ᷴӦ���ӷ���ʽΪFe2O3+6H+=2Fe3++3H2O��
�ʴ�Ϊ��Al2O3+6H+=2Al3++3H2O��Fe2O3+6H+=2Fe3++3H2O��
��2������ټ���������ᣬ��Һ���к���Al3+��Fe3+��H+�ȣ�Ӧ�����������ƣ���ȥ�����ӣ���������ת��ΪAlO2-����ӦΪ H++OH-=H2O��Fe3++3OH-=Fe��OH��3����Al3++4OH-=AlO2-+2H2O��
�ʴ�Ϊ��NaOH�� H++OH-=H2O��Fe3++3OH-=Fe��OH��3��Al3++4OH-=AlO2-+2H2O��
��3����Һ�ں���AlO2-�������ͨ�������̼����AlO2-��Ӧ���ɵij���Ϊ����������
��Ӧ��ѧ����ʽΪNaAlO2+CO2+2H2O=Al��OH��3��+NaHCO3��
�ʴ�Ϊ��NaAlO2+CO2+2H2O=Al��OH��3��+NaHCO3��
��4������NaOH��Һ�ܽ����������в���SiO2�ܽ���NaOH��Һ�����ɹ����ƣ�ͨ�������̼�����ɹ�����������ʹ�����Ƶõ�Al2O3����SiO2���ʣ�
�ʴ�Ϊ������NaOH��Һ�ܽ����������в���SiO2�ܽ���NaOH��Һ�����ɹ����ƣ����ʹ�����Ƶõ�Al2O3����SiO2���ʣ�
������SiO2�������Ӧ��Fe2O3���������Ʋ���Ӧ���������ܷ�Ӧ����ƫ��������ͨ������Ķ�����̼����������������������
�ɹ������̿�֪�����ͨ���Ϊ������̼�����ɵij���Ϊ����������������Һ�ں���AlO2-�������Ӧ�����������ƣ���ȥ�����ӣ���������ת��ΪAlO2-��������Һ���к���Al3+�������ӦΪ�������ᣬ��ȥSiO2��
�����������Կ�ͼ����ʽ�������Ļ���������ʣ��Ѷ��еȣ����ұ������������ԭ�����Ƕ�֪ʶǨ�Ƶ��ۺ����ã�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ