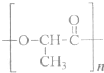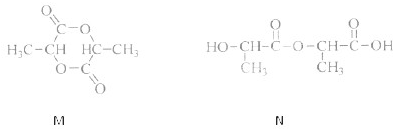��Ŀ����
 ��������ɸ�������ɺ����ʽ��з��࣬���磺������ͼ��ʾ��
��������ɸ�������ɺ����ʽ��з��࣬���磺������ͼ��ʾ����1����ͼ��ʾ�����ʷ����������
��״���෨
��״���෨
����2����K��Na��H��O��S��N�������ֻ�����Ԫ����ɺ��ʵ����ʣ��ֱ������±��Тڡ��ܡ����森
| ������� | �� | �� | �� | ������ | �⻯�� |
| ��ѧʽ | ��H2SO4 �� HNO3 HNO3 |
��NaOH �� KOH KOH |
��Na2SO4 �� Na2SO3 Na2SO3 |
��SO2 ��SO3 |
��NH3 |
2SO2+O2 2SO3
2SO3
 2SO3
2SO32SO2+O2 2SO3
2SO3
�� 2SO3
2SO3��4��д��Cu��ٵ�Ũ��Һ��Ӧ�Ļ�ѧ����ʽ
Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O
| ||
Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O
���÷�Ӧ����������
| ||
������
������
����5��ʵ�����Ʊ��᳣��
�Ȼ��
�Ȼ��
����������
��������
��Ӧ�����������ķ�������ʪ��ĺ�ɫʯ����ֽ���飬��ֽ������
��ʪ��ĺ�ɫʯ����ֽ���飬��ֽ������
����������1����״���෨��һ�ֺ�����ķ��෨�����ղ�Σ�һ��һ�����֣�����һ�ô�����
��2����������������ȫ���������ӵĻ������������������ȫ��Ϊ���������ӣ��ε������������Ϊ�������ӣ�������Ϊ������ӣ�
��3���������������������������
��4��ͭ��Ũ���ᷴӦ��������ͭ�����������ˮ�����ϼ۽��͵ķ�Ӧ��Ϊ��������
��5���Ȼ�狀��������ƹ��������ȡ������������ʹʪ��ĺ�ɫʯ����ֽ������
��2����������������ȫ���������ӵĻ������������������ȫ��Ϊ���������ӣ��ε������������Ϊ�������ӣ�������Ϊ������ӣ�
��3���������������������������
��4��ͭ��Ũ���ᷴӦ��������ͭ�����������ˮ�����ϼ۽��͵ķ�Ӧ��Ϊ��������
��5���Ȼ�狀��������ƹ��������ȡ������������ʹʪ��ĺ�ɫʯ����ֽ������
����⣺��1����״���෨��һ�ֺ�����ķ��෨�����ղ�Σ�һ��һ�����֣�����һ�ô�������Ҷ��֦���ˡ�����ͼʾ����������״ͼ��
�ʴ�Ϊ����״���෨��
��2����������������ȫ���������ӵĻ������HNO3����������������ȫ��Ϊ���������ӣ���KOH���ε������������Ϊ�������ӣ�������Ϊ������ӣ���Na2SO3��
�ʴ�Ϊ����HNO3 ��KOH ��Na2SO3��
��3�����������������������������ʽΪ��2SO2+O2 2SO3��
2SO3��
�ʴ�Ϊ��2SO2+O2 2SO3��
2SO3��
��4��ͭ��Ũ���ᷴӦ��������ͭ�����������ˮ������ʽΪ��Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O������H2SO4����Ļ��ϼ۽��ͣ�����������
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O����������
��5��ʵ������ȡ�����Ļ�ѧ����ʽ�ǣ�2NH4Cl+Ca��OH��2
2NH3��+CaCl2+2H2O��������ʹʪ��ĺ�ɫʯ����ֽ������
�ʴ�Ϊ���Ȼ�李��������ƣ���ʪ��ĺ�ɫʯ����ֽ���飬��ֽ������
�ʴ�Ϊ����״���෨��
��2����������������ȫ���������ӵĻ������HNO3����������������ȫ��Ϊ���������ӣ���KOH���ε������������Ϊ�������ӣ�������Ϊ������ӣ���Na2SO3��
�ʴ�Ϊ����HNO3 ��KOH ��Na2SO3��
��3�����������������������������ʽΪ��2SO2+O2
 2SO3��
2SO3���ʴ�Ϊ��2SO2+O2
 2SO3��
2SO3����4��ͭ��Ũ���ᷴӦ��������ͭ�����������ˮ������ʽΪ��Cu+2H2SO4��Ũ��
| ||
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
| ||
��5��ʵ������ȡ�����Ļ�ѧ����ʽ�ǣ�2NH4Cl+Ca��OH��2
| ||
�ʴ�Ϊ���Ȼ�李��������ƣ���ʪ��ĺ�ɫʯ����ֽ���飬��ֽ������
���������⿼�������ʵķ���������ʵ��Ʊ����ʼ��飬�漰�˻�ѧ����ʽ����д�������Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ