��Ŀ����
ijУ��ѧС���ͬѧ��һ����������·������õ�Cu��Al��Fe������Au��Pt�Ƚ����Ļ���������������Ʊ�ǿ��ͭ������������ķ�����
�ش��������⣺
��1���ڢڲ�����H2O2��Ϊ�˳���Fe2+���÷�Ӧ�����ӷ���ʽΪ_________��
��2������2����Ҫ�ɷ���Fe(OH)3��Al(OH)3��������2��ȡAl2(SO4)3��18H2Oʵ����̵��������__________________��
��3���õڢ۲�����CuSO4��5H2O���Ƶ�Cu(OH)2����ѧ�С��Ϊ̽��Cu(OH)2���ȷֽ���P�������ʣ��������ʵ����̣�ȡ0.98g Cu(OH)2������ȣ���ͭ�����������ɣ����������¶ȱ仯��ͼ��ʾ������A��B�Ļ�ѧʽ�ֱ�Ϊ____��Cu2O��ͨ������ʵ���ͼ����Եó����½��ۣ�����ʱB______������ȶ������ȶ�������
�С��ͬѧ������������ʵ�飺
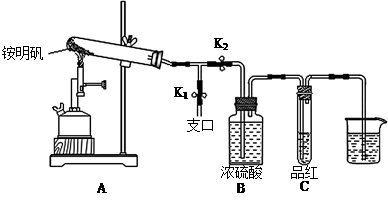
�ټ�ͬѧ������װ�ý���ʵ�飨�г�װ��ĩ�����������յó��Ľ����Ǽ���ʱA�ɽ�NH3����ΪN2��A����ԭΪ����Cu��֧�ּ�ͬѧ���۵�������______��
����ͬѧ������ʵ��װ�ý���ʵ�飬ʵ���й۲쵽��Ϸ�ĩ���ձ�ɺ�ɫ���ʣ�ͬʱ����һ����ɫ���д̼�����ζ�����壬�����廯ѧʽΪ_______��������Ϊ��ͬѧ��ʵ��װ������װB���ʵ��Թ����ձ�֮�����һ��������װ�ã�����Ϊ�Ƿ��б�Ҫ_____�����б�Ҫ�����ԭ���ԸĽ�____________��
��15�֣���1��2Fe2����H2O2��4OH����2Fe(OH)3��2�֣�
��2�����ˡ�ϴ�ӡ����2�֣�ֻ���һ�����÷֣���2����1�֣���3��CuO��2�֣������ȶ���2�֣�
��Ӳ�ʲ������к�ɫ�����ɺ�ɫ���ձ��е��ܿڲ��ֲ������ݣ�����ɫ����ζ�������ɣ�2�֣�
��SO2��2�֣�����Ҫ��1�֣���ʵ������Ϊ��Ӧ����SO2��������NaOH��Һ��Ӧ����ʹ��ϵ��ѹǿ�����С��ɵ�����1�֣����ڵ���ĩ������һ���õ�©����1�֣���©����Ե�ո�û��Һ���£�1�֣�������ĩ������һ����ܣ��ڸ���ܵü��첿�ֲ���Һ���£����������Ҳ�÷֣�
���������������1���������Ӿ��л�ԭ�ԣ�˫��ˮ���������ԣ��ڼ��������£�������������������������������ȥ����Ӧ�����ӷ���ʽ��2Fe2����H2O2��4OH����2Fe(OH)3��
��2������������������������������������������������Ʊ�����������Ҫ�����ǣ�����2�ܽ�������������Һ�����˵õ������������壬Ȼ������Һ�н���������ϡ����������������Ȼ������Ũ������ȴ�ᾧ���ɣ�����ʵ����̵�������ǹ��ˡ�ϴ�ӡ����
��3��A�����������0.80g������ڼ��ȹ����й������0.98g��0.80g��0.18g������0.98g������ͭ��ˮ�������� ��18g/mol��0.18g����˵��A����ǡ��������ͭ����ѧʽ��CuO������ͭ��������������������ͭ����˵���ڸ����£�������ͭ������ͭ���ȶ���
��18g/mol��0.18g����˵��A����ǡ��������ͭ����ѧʽ��CuO������ͭ��������������������ͭ����˵���ڸ����£�������ͭ������ͭ���ȶ���
������ͭ�ɽ�NH3����ΪN2��ͬʱ����ͭ����ԭΪ����Cu������֧�ּ�ͬѧ���۵�������Ӳ�ʲ������к�ɫ�����ɺ�ɫ���ձ��е��ܿڲ��ֲ������ݣ�����ɫ����ζ�������ɡ�
�����ڸ���������ɫ���д̼�����ζ�����壬���Ը������ʵ����Ԫ�ؿ�֪����������SO2������ʵ������Ϊ��Ӧ����SO2��������NaOH��Һ��Ӧ����ʹ��ϵ��ѹǿ�����С��ɵ����������ڵ���ĩ������һ���õ�©����©����Ե�ո�û��Һ�����Է�ֹ�������������б�Ҫ�ġ�
���㣺����������ԭ��Ӧ����ʽ�����ʵ��Ʊ��ڷ��룻������ͭ�ֽ�����Լ�ͭ���������ȶ����жϣ�����ͭ��SO2�����ʡ�����װ�õ�ѡ���

 Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д� ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д�(4��)����(������Ϊ����)
| ��� | ���� | �����Լ� |
| ��1�� | CO2��HCl�� | |
| ��2�� | Fe2O3��Al2O3�� | |
| ��3�� | NaCl��Һ��MgCl2�� | |
| ��4�� | NO���壨NO2�� | |
��9�֣�����ֲ��纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڡ�ʵ������Ӻ�������ȡ����������£�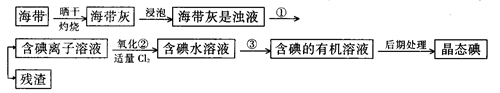
��1��ָ����ȡ��Ĺ������йص�ʵ��������ƣ�
�� ����______________________��
д��ʵ������йط�Ӧ�����ӷ���ʽ _______________________ ��
��2����ȡ��Ĺ����У��ɹ�ѡ����й��Լ���___________��
| A���ױ����ƾ� | B�����Ȼ�̼���� |
| C�����ͣ����� | D�����ͣ����� |
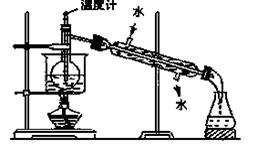
��3���Ӻ�����л���Һ����ȡ��ͻ����л��ܼ����辭������ָ����ͼʵ��װ���еĴ���֮������ �� �� ��
��4�����������������ʱ��ʹ��ˮԡ��ԭ����___________�����̬����___________��ۼ���
��14�֣�ijѧ��Ϊ�ⶨij�ռ���Ʒ��NaOH��������������������ʵ�飨��֪����Ʒ�к�������Na2CO3���ʣ�
a����250mL������ƿ�ж��ݣ����Ƴ�250mL�ռ���Һ��
b���ü�ʽ�ζ�����ȡ25.00mL�ռ���Һ����ƿ�У���������BaCl2��ҺʹNa2CO3��ȫת���BaCO3����뼸�η�ָ̪ʾ����
c������ƽ��ȷ��ȡ�ռ���Ʒ10.5g�����ձ���������ˮ�ܽ⣻
d�������ʵ���Ũ��Ϊ1.000mol/L�ı�������Һװ����ʽ�ζ����У�����Һ�棬���¿�ʼʱ�Ķ���Ȼ��ʼ�ζ���
e������ƿ�µ�һ�Ű�ֽ���ζ�����Һǡ�ñ�Ϊ��ɫΪֹ�����¶���������գ�
��1����ȷ���������˳���ǣ�����ĸ��գ�_____��_____��_____��_____��_______.
��2����ʽ����ʽ���ζ�����ʹ��ǰ����еĵ�һ��������____________����ѧ��ѧʵ�鳣��������ʹ��ǰ�͵ζ���ʹ������ͬ�����IJ�ͬ�ಣ���������� ��_______��
��3���ظ������ζ���������¼�������£�
| ʵ���� | ����Һ��H2SO4��(aq) Ũ�ȣ�mol/L�� | �ζ����ʱ���� ���V��mL�� | ������Һ��NaOH��(aq) ���V��mL�� |
| 1 | 1.000 | 11.00 | 25.00 |
| 2 | 1.000 | 12.04 | 25.00 |
| 3 | 1.000 | 12.18 | 25.00 |
������ʵ���У����в���������������ȷ������ɽ��ƫ�͵���__________________��
A��a��������δ����Һ��ȴ�����¾�ת�Ƶ�����ƿ�ж��ݡ�
B��c�������У�����ҩƷʱ������������̣�NaOH�������̡�
C���ζ��յ����ʱ���Ӷ�����
D����ʽ�ζ���ʹ��ǰ��δ�ñ�H2SO4��Һ��ϴ��
E����ƿˮϴ��δ�����ֱ��ʢ������Һ��
����ͬѧ�����ԭʵ�鷽���еķ�ָ̪ʾ����Ϊ����ָʾ��������Ϊ�Ƿ���У�________������С������С�����������ü���Ϊָʾ������ⶨ�����Σ�________���ƫ�ߡ� ��ƫ�͡�����ȷ����



 ��
��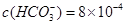
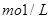 ��Ҫ����10 m3������Ȼˮ�����ȼ���Ca��OH��2 g���ټ���Na2CO3 g��
��Ҫ����10 m3������Ȼˮ�����ȼ���Ca��OH��2 g���ټ���Na2CO3 g��