��Ŀ����
����ͭ��һ����Ҫ�Ļ���ԭ�ϣ���ҵ�ϳ�������Ϊԭ�����Ʊ�����ͭ����1����ҵ��������������У�����������ʱ�����ķ�����һ�ֶ�����Դ��Ϊ�˴ӷ����д�ѡ���Ʒλ�ϸ����������������COʹ������Fe2O3ת��Ϊǿ����Fe3O4��д���÷�Ӧ�Ļ�ѧ����ʽ
ʵ�鷢�֣�CO̫���̫�ٶ��ᵼ�´�������ʽ��ͣ�ԭ����
��2���ⶨ����ͭ�����нᾧˮ������ʵ�鲽��Ϊ��
����1��ȷ����һ���ྻ�������������
����2����һ����������ͭ����������ϸ���������г��أ�
����3����ʢ���������������ȣ��������ɰ�ɫ��ĩʱ��ֹͣ���ȣ�
����4��������3�е������������������ȴ�����º��أ�
����5��
����6������ʵ�����ݼ�������ͭ���������нᾧˮ������������
�����ʵ�鲽��5��
��3����֪����ͭ�������ȿ�����ʧȥ�ᾧˮ���¶��������Էֽ�����ͭ�������ȡ25.0g CuSO4?5H2O ����������ȣ�����������1 200�沢����1Сʱ��ʵ���ù�������ʣ�
| ʣ���������� | ԭʼ�������� |

��110��ʱ���ù���ijɷ�Ϊ
��1 200�沢����1Сʱ����Ӧ������̬�����ȥˮ�����ʵ���Ϊ
A��0mol B��0.1mol C��0.125mol D������0.125mol��
��������1�����ݸ���������COʹ������Fe2O3ת��Ϊǿ����Fe3O4�����������غ�ȷ�����ﲢ��ƽ��CO̫�ർ��Fe3O4��ԭΪ����CO̫�٣�Fe2O3ת����Fe3O4��ת���ʲ��ߣ�
��2������ֱ�����γ������������0.1gȷ����ȫʧˮ��
��3����ͼʾ���ݿ�֪��ʧȥˮ������Ϊ��25.0g��14.4%=3.6g�����ݹ�ϵʽ��CuSO4?5H2O��CuSO4?XH2O���ò��������X���Ӷ��ó���ѧʽ��������Ŀ��Ϣ����ͭ�ֽ�����ͭ����������ݻ��ϼ�������֪���ﻹ��SO2��O2���ֽⷽ��ʽ��2CuSO4
2CuO+2SO2��+O2����Ȼ����㣮
��2������ֱ�����γ������������0.1gȷ����ȫʧˮ��
��3����ͼʾ���ݿ�֪��ʧȥˮ������Ϊ��25.0g��14.4%=3.6g�����ݹ�ϵʽ��CuSO4?5H2O��CuSO4?XH2O���ò��������X���Ӷ��ó���ѧʽ��������Ŀ��Ϣ����ͭ�ֽ�����ͭ����������ݻ��ϼ�������֪���ﻹ��SO2��O2���ֽⷽ��ʽ��2CuSO4
| ||
����⣺��1��COʹ������Fe2O3ת��Ϊǿ����Fe3O4��CO2������ʽΪ��3Fe2O3+CO
2Fe3O4+CO2��CO̫�ർ��Fe3O4��ԭΪ����CO̫�٣�Fe2O3ת����Fe3O4��ת���ʲ��ߣ�����CO̫���̫�ٶ��ᵼ�´�������ʽ��ͣ�
�ʴ�Ϊ��3Fe2O3+CO
2Fe3O4+CO2��CO̫�ർ��Fe3O4��ԭΪ����CO̫�٣�Fe2O3ת����Fe3O4��ת���ʲ��ߣ�
��2��������4�е������ټ���һ��ʱ�䣬�������������ȴ�����º��أ��ظ���������ֱ�����γ������������0.1g��
�ʴ�Ϊ��������4�е������ټ���һ��ʱ�䣬�������������ȴ�����º��أ��ظ���������ֱ�����γ������������0.1g��
��3��CuSO4?5H2O��CuSO4?XH2O��m
250 18��5-x��
25.0g 3.6g
��
=
��
��ã�X=3��������110��ʱ���ù���ijɷ�ΪCuSO4?3H2O��
25.0g CuSO4?5H2O�����ʵ���Ϊ��
���� CuSO4�����ʵ���Ϊ0.1mol��
2CuSO4
2CuO+2SO2��+O2��
0.1mol 0.1mol 0.05mol
��Ӧ������̬�����ȥˮ�����ʵ���Ϊ0.15mol��
�ʴ�Ϊ��CuSO4?3H2O��D��
| ||
�ʴ�Ϊ��3Fe2O3+CO
| ||
��2��������4�е������ټ���һ��ʱ�䣬�������������ȴ�����º��أ��ظ���������ֱ�����γ������������0.1g��
�ʴ�Ϊ��������4�е������ټ���һ��ʱ�䣬�������������ȴ�����º��أ��ظ���������ֱ�����γ������������0.1g��
��3��CuSO4?5H2O��CuSO4?XH2O��m
250 18��5-x��
25.0g 3.6g
��
| 250 |
| 25.0g |
| 18(5-x) |
| 3.6g |
��ã�X=3��������110��ʱ���ù���ijɷ�ΪCuSO4?3H2O��
25.0g CuSO4?5H2O�����ʵ���Ϊ��
| 25.0g |
| 250g/mol |
2CuSO4
| ||
0.1mol 0.1mol 0.05mol
��Ӧ������̬�����ȥˮ�����ʵ���Ϊ0.15mol��
�ʴ�Ϊ��CuSO4?3H2O��D��
������������Ҫ����������ͭ�����нᾧˮ�����IJⶨ������ͭ����ֽ�����ȷ����ע���������ʹ�úͻ�ѧ���㣬�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�
�����Ŀ






 ������ĿΪ ��
������ĿΪ �� ����CuSO4��5H2O��д��[Cu(H2O) 4]SO4
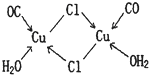
��H2O����ṹʾ��ͼ���£�
����CuSO4��5H2O���[Cu(H2O) 4]SO4
��H2O����ṹʾ��ͼ���£�
 ������ĿΪ
��
������ĿΪ
��