��Ŀ����
����Ŀ��������ˮ�п��ܴ��ڵ���ƽ�⡢�ε�ˮ��ƽ��ͳ������ܽ�ƽ�⣬���Ƕ��ɿ�����ѧƽ�⡣�������ѧ��֪ʶ�ش�
��1��0.1 mol��L��1��NH4Cl��Һ���ڸ���Һ�и������ӵ�Ũ���ɴ�С˳��Ϊ_________��
��2��0.1 mol��L��1 NaHCO3��Һ�������NaHCO3��Һ�Լ��Ե�ԭ��________��
��3��FeCl3��Һ��ʵ����������FeCl3��Һʱ������____________��������ˮ�⡣
��4������AgI����ı�����Һ�У�
�ټ������AgNO3����c(I��)_____(������ ��С�����䡱����ͬ)��c(Ag��)__��
�����ļӸ����AgI����c(Ag��)��____��
�����ļ�Na2S���壬��c(I��)____��c(Ag��)_____��
���𰸡�c(Cl-)>c(NH4+)> c(H+)>c(OH-) HCO3-��ˮ�����HCO3-�ĵ��� HCl ��С ��� ���� ��� ��С
��������
��1���Ȼ����Һ��笠���������ˮ�⣬ʹ��Һ�������ԣ�
��2��HCO3������ˮ��ƽ��͵���ƽ�⣬��ˮ��ƽ��Ϊ����
��3��FeCl3��Һ�У�����������ˮ��ʹ��Һ�����ԣ�
��4�����ݳ����ܽ�ƽ����ƶ����͡�
(1) 0.1 mol��L��1��NH4Cl��Һ��笠���������ˮ��,ˮ�����ӷ���ʽΪNH4++H2O![]() NH3H2O+H+����Һ�е�����Ũ�ȴ�СΪ: c(Cl-)>c(NH4+)> c(H+)>c(OH-)��
NH3H2O+H+����Һ�е�����Ũ�ȴ�СΪ: c(Cl-)>c(NH4+)> c(H+)>c(OH-)��
��: c(Cl-)>c(NH4+)> c(H+)>c(OH-)
(2) 0.1 mol��L��1 NaHCO3��Һ�д���̼��������ӵ�ˮ��ƽ��Ϊ:HCO3��+H2O![]() H2CO3+OH��,����ƽ��Ϊ: HCO3��
H2CO3+OH��,����ƽ��Ϊ: HCO3��![]() CO32-+H+��HCO3����ˮ��̶ȴ��ڵ���̶���Һ,���Լ��ԣ�
CO32-+H+��HCO3����ˮ��̶ȴ��ڵ���̶���Һ,���Լ��ԣ�
�𰸣�HCO3-��ˮ�����HCO3-�ĵ���
(3)ʵ����������FeCl3��Һʱͨ����Ҫ�����м���������Fe3+ˮ�⣻
�𰸣�HCl
��4��������AgI����ı�����Һ�д���AgI��s��![]() Ag+��aq��+I-��aq���У��������AgNO3��������c��Ag+����ƽ�������ƶ�����c(I��) ��С��c(Ag��)��δ����AgNO3ʱ���
Ag+��aq��+I-��aq���У��������AgNO3��������c��Ag+����ƽ�������ƶ�����c(I��) ��С��c(Ag��)��δ����AgNO3ʱ���
�𰸣���С ���
������AgI����ı�����Һ�У����ļӸ����AgI����Ϊ�¶�һ��ʱ������AgI��Һ�У�����Ũ��һ����c(Ag��)�����䣻
�𰸣�����
������AgI����ı�����Һ�д���AgI��s��![]() Ag+��aq��+I����aq���У��ļ�Na2S���壬ӦΪAg+��S2-��Ӧ����Ag2S�����������ܽ�ƽ�������ƶ�����c(I��)���c(Ag��)��С��
Ag+��aq��+I����aq���У��ļ�Na2S���壬ӦΪAg+��S2-��Ӧ����Ag2S�����������ܽ�ƽ�������ƶ�����c(I��)���c(Ag��)��С��
�𰸣���� ��С

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
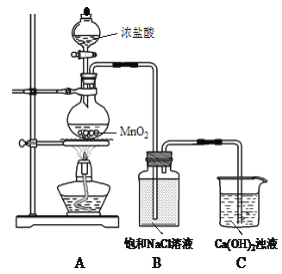
Сѧ��10����Ӧ����ϵ�д�����Ŀ������ˮ�ܿ���Ҫ�ɷ�ΪCo2O3��������Fe2O3��Al2O3��MnO��MgO��CaO��SiO2�ȣ�������ȡ���ֻ����Լ�������Ϊ�����ܾ�����Ȼ��ܾ�����Ʊ����̣��ش��������⣺
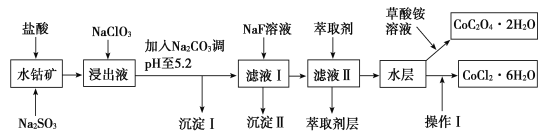
��֪���ٽ���Һ�к��е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Al3+��Mg2+��Ca2+�ȡ�
�ڳ�������ֻ�������ֳ�����
�������в���������������������ʽ����ʱ��Һ��pH������
������ | ��ʼ���� | ��ȫ���� |
Fe(OH)3 | 2.7 | 3.7 |
Fe(OH)2 | 7.6 | 9.6 |
Co(OH)2 | 7.6 | 9.2 |
Al(OH)3 | 4.0 | 5.2 |
Mn(OH)2 | 7.7 | 9.8 |
��1�������������������뻹ԭ�����ʵ���֮��Ϊ___��
��2��NaClO3�ڽ���Һ�з�����Ӧ�����ӷ���ʽΪ___��
��3������Na2CO3��pH��5.2��Ŀ����___����ȡ���㺬��Ԫ�أ�����������Ҫ�ɷ�Ϊ__��
��4���������������ˮ�����Ũ�������pHΪ2��3��___��___�����ˡ�ϴ�ӡ���ѹ��ɵȹ��̡�
��5��Ϊ�ⶨ�ֲ�Ʒ��CoCl2��6H2O�ĺ�������ȡһ�������Ĵֲ�Ʒ����ˮ���������������ữ����������Һ�����ˡ�ϴ�ӡ���������������ͨ�����㷢�ֲִ�Ʒ��CoCl2��6H2O������������100%����ԭ�������___���ش�һ��ԭ�ɣ���
��6����5.49g�����ܾ���(CoC2O4��2H2O)���ڿ����м��ȣ����ȹ����в�ͬ�¶ȷ�Χ�ڷֱ�õ�һ�ֹ������ʣ������������
�¶ȷ�Χ/�� | 150��210 | 290��320 |
��������/g | 4.41 | 2.41 |
���ⶨ���������ȹ��̣�ֻ����ˮ������CO2���壬��290��320���¶ȷ�Χ��ʣ��Ĺ������ʻ�ѧʽΪ___��[��֪��CoC2O4��2H2O��Ħ������Ϊ183g��mol-1]