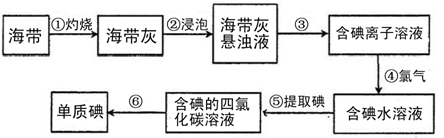
��Ŀ����
�����к��зḻ�ĵ⡣Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺
����д���пհף�
(1)��������պ���ʱ������Ҫ���ż��⣬����Ҫ�õ���ʵ��������_____________��������������ѡ��������������ñ����ĸ��д�ڿհ״�����
A.�ձ� B.���� C.������ D.������ E.�ƾ��� F.������
(2)����۵�ʵ�����������_____________;�����Ŀ���ǴӺ��ⱽ��Һ�з�������ʵ�ͻ��ձ����ò����ʵ�����������_____________��
(3)����ܷ�Ӧ�����ӷ���ʽ��_____________________________________________��
(4)������У�ijѧ��ѡ���ñ�����ȡ���������____________________________________��
(5)�����һ�ּ�����ȡ����ˮ��Һ���Ƿ��е��ʵ�ļ�����_________________��
���������⿼��I-��I2���������ʺͻ�ѧ�����Լ���ص�ʵ�������I-���к�ǿ�Ļ�ԭ�ԣ�I2���������ڱ������Ȼ�̼���л��ܼ����������۱���ɫ��
�𰸣�(1)BDE (2)���� ���� (3)2I-+MnO2+4H+![]() Mn2++I2+2H2O (4)����ˮ��������;���ڱ��е��ܽ�ȱ���ˮ�д� (5)ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ;�۲��Ƿ������ɫ(���������˵�����е��ʵ�)
Mn2++I2+2H2O (4)����ˮ��������;���ڱ��е��ܽ�ȱ���ˮ�д� (5)ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ;�۲��Ƿ������ɫ(���������˵�����е��ʵ�)

 53���ò�ϵ�д�
53���ò�ϵ�д�