��Ŀ����
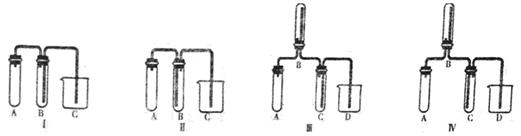
��ͼ����ʵ�����Ƶõ���ϩ(C2H5OH  CH2=CH2��+H2O)����ˮ������ȡ1,2-��������IJ���װ��ͼ������ͼʾ�ж�����˵����ȷ����( )
CH2=CH2��+H2O)����ˮ������ȡ1,2-��������IJ���װ��ͼ������ͼʾ�ж�����˵����ȷ����( )
| A��װ�âٺ�װ�â��ж�ʢ��ˮ����������ͬ |
| B��װ�âں�װ�â��ж�ʢ��NaOH��Һ�������յ�������ͬ |
| C��������÷�Һ�ķ������з��룬1,2-��������Ӧ�ӷ�Һ©�����Ͽڵ��� |
| D���Ʊ���ϩ������1,2-��������ķ�Ӧ���ͷֱ�����ȥ��Ӧ�ͼӳɷ�Ӧ |
D
�������������װ�â��е�ˮ�Ǽ������Ƿ������װ�â�����ȴ��ʹ1,2-��������Һ����A��ȷ��װ�âں�װ�â��ж�ʢ��NaOH��Һ���ֱ�����SO2��Br2����B����ȷ��1,2-����������ˮ���²㣬Ӧ�ӷ�Һ©�����¿ڷų���C����D��ȷ��
���㣺�л�����Ʊ�װ�õ�ԭ�������뷽����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�CuSO4��5H2O��ͭ����Ҫ��������Ź㷺��Ӧ�á�ʵ�����ô�ͭ�������������Ʊ�CuSO4��5H2O���������£�
�ش��������⣺
��1��ʵ��������250 mL4.8 mol��L��1��ϡ���ᣬ����IJ�������������������Ͳ���ձ������Ҫ__________________________________________________��
��2����֪��ͬ����������������������������pH���±���
| ���� | Fe3+ | Cu2+ | Fe2+ |
| ��ʼ������pH | 2.7 | 4.4 | 7.0 |
| ������ȫ��pH | 3.7 | 6.4 | 9.6 |
��ȥFe3+����Һ��pHӦ����_______________��Χ����pH��ѡ�õ��Լ���____________��
��3�����Ƶõ�CuSO4��5H2O�д�������Cu(NO3)2����һ���ᴿ��ʵ�����������__________��
��4����õ�CuSO4��5H2O������渽���������ӣ����þƾ�ϴ�ӵ�ԭ����____________��
�ñ���BaCl2��Һ�ⶨ����Ĵ���ʱ�����ִ��ȴ���100%���ⶨ�����в��������ɺ��ԣ��������ԭ����_____________________________________________����дһ�֣���
��5��ʵ��������ȡ����NO���塣��ͼ�����ʺϵ�ʵ��װ����__________������ţ�����ע��װ��������ҩƷ�����ơ�
A��__________��B��__________��C��__________��D��__________��������ѡ���װ�ÿ�������Ҳ���Բ�������
����ʵ�鷽������У����е���( )
| A����ϡ�������ˣ���ȥ����ͭ���е�����þ�ۺ����� |
| B������ȡ�ķ����������ͺ�ú�� |
| C�����ܽ⡢���˵ķ�����������غ��Ȼ��ƹ���Ļ���� |
| D���������������Ļ������ͨ�����ȵ�����ͭ���Գ�ȥ���е����� |
�����й�ʵ��װ�ý��е���Ӧʵ�飬���ܴﵽʵ��Ŀ�ĵ���
| A����װ�âٳ�ȥCl2�к��е�����HCl |
| B����װ�âڴ�KCl��Һ�л��KCl���� |
| C����װ�â۳�ȥO2�л��е�һ����SO2 |
| D����װ�â���ʵ������ȡ���ռ����� |
����ʵ�鲻�ܴﵽĿ�ĵ���
| A���ñ���NaHCO3��Һ��ȥCO2�л��е�HCl |
| B���ü�������CuCl2��Һ�ķ����Ʊ���ˮCuCl2���� |
| C���÷�Һ©������CCl4��ȡ��ˮ���ѷֲ���л����ˮ�� |
| D���ü��ȷֽ�ķ�������̼���ƺ�̼���������ֹ��� |
�����к���Ԫ�ء��Ӻ�������ȡ�������²���:������Һ��ͨ������Cl2���ڽ��������ճɻҺ��ˮ���裻�ۼ�CCl4�����÷�Һ©����ҹ���ݹ��ˡ������IJ���˳��
| A���٢ڢۢܢ� | B���ڢݢ٢ۢ� | C���٢ۢݢڢ� | D���ڢ٢ۢݢ� |
����һ������ˮ��Һ��ֻ���ܺ������������е������֣�K����NH4����Cl����Mg2����Ba2����CO32����SO42������ȡ����100mL��Һ��������ʵ�飺
��1����һ�ݼ���AgNO3��Һ�г�������
��2���ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.04mol
��3�������ݼ�����BaCl2��Һ�ø������6.27g������������ϴ�ӡ��� ���������Ϊ2.33g����������ʵ�飬�����Ʋ���ȷ����
| A��Cl�������ܴ��� | B��100mL��Һ�к�0.01mol CO32�� |
| C��K��һ������ | D��Ba2��һ�������ڣ�Mg2�����ܴ��� |

