��Ŀ����
����Ŀ���±�Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�ء�

��1������λ��ds����Ԫ����________�����ţ���д����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽ��___________��
��2���������γɵĻ���������У�����ԭ�ӵ��ӻ��������Ϊ________�������γɵĻ�����ľ���������________��
��3����������������Ԫ���У���һ������������________�����ţ���
��4��Ԫ�����ļ���̬�⻯����ˮ�е��ܽ�ȱ�ͬ������Ԫ�ص��⻯���ܶ��ԭ����____________________________________________________________________________________��
��5��������γɵľ�����۵���ڢ�����γɵľ�����۵㣬ԭ��____________________________��

��6��1183K���£�Ԫ�����ĵ���Ϊ�����������壨�����ṹ��ͼ1����1183K����ת��Ϊ�����������壨�����ṹ��ͼ2������ͼ2�У���һ��ԭ�Ӿ����������ȵ�ԭ�Ӹ���Ϊ________��ͼ1��ͼ2��ʾ�����к��е�ԭ�Ӹ���֮��Ϊ________��
���𰸡��� ![]() ��
��![]() sp ���Ӿ��� �� ��������ˮ����֮����γ�����ҷ�����ѧ��Ӧ
sp ���Ӿ��� �� ��������ˮ����֮����γ�����ҷ�����ѧ��Ӧ ![]() �İ뾶��
�İ뾶��![]() ��С��ʹ������þ����ľ����ܴ�����þ���� 12 1:2
��С��ʹ������þ����ľ����ܴ�����þ���� 12 1:2
��������
��Ԫ�������ڱ���λ�ÿ�֪����ΪBe����ΪC����ΪN����ΪO����ΪMg����ΪAl����ΪS����ΪCl����ΪFe����ΪCu��
��1��Ԫ�����ڱ���λ��ds����Ԫ��Ϊ�ڢ�B����B�壬�ʷ��ϵ�Ϊ��Ԫ��ͭ����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ[Ar]3d104s1��
�ʴ�Ϊ�⣻[Ar]3d104s1��![]() ��
��
��2���ٺ͢��γɵĻ�����ΪBeCl2��Be�����¶�ԭ�ӣ��γ�2�����۵�����������ԭ�ӵ��ӻ��������Ϊsp�ӻ����͢��γɵĻ�����ΪAlCl3���ɷ��ӹ��ɣ�Ϊ���Ӿ��壬
�ʴ�Ϊsp�����Ӿ��壻
��3��Nԭ�ӵ�2p���Ӱ���Ϊ�ȶ��ṹ�����һ�����ܴڡ��ۡ�������Ԫ���У���һ�����������Ǣڣ�
�ʴ�Ϊ�ڣ�
��4��Ԫ�آ۵ļ���̬�⻯��Ϊ����������ˮ�����γ��������Ԫ�آ۵ļ���̬�⻯����ˮ�е��ܽ�ȱ�ͬ������Ԫ�ص��⻯���ö��ԭ���Ƿ���֮���γ������
�ʴ�Ϊ����֮���γ������
��5��������γɵľ��壨MgO�����۵���ڢ�����γɵľ��壨MgS���۵㣬ԭ��Ϊ�����Ӱ뾶С�������Ӱ뾶����MgO�����Ӽ��̣����ܴ�
�ʴ�Ϊ�����Ӱ뾶С�������Ӱ뾶����MgO�����Ӽ��̣����ܴ�
��6����ͼ1��֪��������ԭ�Ӿ��������ԭ��λ�ڶ��㣬����8������ͼ2��֪���붥��ԭ�Ӿ��������ԭ��λ�����ģ�1����������3�����ռ���8��������϶���ã���1���汻2���������ã�����һ��ԭ�Ӿ����������ȵ�ԭ�Ӹ���Ϊ![]() =12����ͼ1��ͼ2��ʾ�����к��е�ԭ�Ӹ���֮��Ϊ��1+8��
=12����ͼ1��ͼ2��ʾ�����к��е�ԭ�Ӹ���֮��Ϊ��1+8��![]() ������6��
������6��![]() +8��
+8��![]() ��=1��2��
��=1��2��
�ʴ�Ϊ12��1��2��

����Ŀ����Ӧ��![]()
![]()
��![]()
![]()
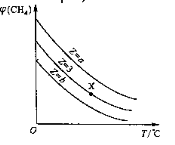
�ڲ�ͬ�¶�ʱ![]() ��
��![]() ��ֵ�����
��ֵ�����
�¶� |
|
|
973 |
|
|
1173 |
|
|
�ݴ˿�֪��Ӧ�ۣ���![]()
![]() ���������Ϣ��ȷ���ǣ� ��
���������Ϣ��ȷ���ǣ� ��
A.![]()
B.�÷�Ӧ����ӦΪ���ȷ�Ӧ
C.�÷�Ӧ����ʱ���Ϸ����![]() ���������
���������![]() ��ת���ʺͷ�Ӧ����
��ת���ʺͷ�Ӧ����
D.�ں������ݻ��ɱ�������з�����Ӧ�ۣ�ѹ�������Ӧ���ʻ�����![]() Ҳ����
Ҳ����
����Ŀ��(1)12g̼������ˮ������Ӧ����CO��H2��������131.3KJ�������˷�Ӧ���Ȼ�ѧ����ʽΪ______________________________��
(2)��֪���µ��Ȼ�ѧ��Ӧ����ʽ��
��2CO��g��+O2��g����2CO2��g�� ��H����566kJ/mol
��2H2��g��+O2��g����2H2O��g�� ��H����484kJ/mol
��CH4��g��+2O2��g����CO2��g��+2H2O��g�� ��H����890kJ/mol
��CH4��g��+CO2��g����2CO��g��+2H2��g�� ��H��______��
(3)��֪H2(g)+Br2(l)=2HBr(g) ��H=��102kJ/mol������������������±���
H2(g) | Br2(l) | HBr(g) | |
1mol�����еĻ�ѧ������ʱ��Ҫ���յ�����/kJ | 436 | a | 369 |
�����aΪ_______��