��Ŀ����
7����֪B��E�ֱ���������ȩ��֬���л���������Ҫԭ�ϣ���ҵ������B��E��һ��;���ǣ�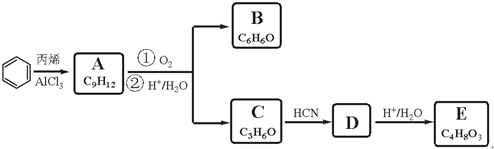
��AΪ����ͬϵ���һ�ȴ�����5�֣�
��C��D��E�ĺ˴Ź�������ͼ�У���ĸ����ֱ�Ϊ1��2��3��
�۵�����E�ֱ���������Na��������NaHCO3��Һ��Ӧ������ͬ�����£����ɵ�����������ͬ��
�ش��������⣺
��1��C���������������ŵ��������ʻ���
��2��C��D�ķ�Ӧ�����Ǽӳɷ�Ӧ��
��3��A�Ľṹ��ʽΪ
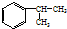 ��A�ĺ��б�����ͬ���칹�壨��A�⣩��7�֣�
��A�ĺ��б�����ͬ���칹�壨��A�⣩��7�֣���4����ȩ��
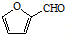 ����һ����Ҫ�Ļ���ԭ�ϣ���ͬ��ȩ�뱽�ӷ�Ӧ���ɷ�ȩ��֬����Ҳ���뱽�ӷ�Ӧ���ɿ�ȩ��֬��д���÷�Ӧ�Ļ�ѧ����ʽ
����һ����Ҫ�Ļ���ԭ�ϣ���ͬ��ȩ�뱽�ӷ�Ӧ���ɷ�ȩ��֬����Ҳ���뱽�ӷ�Ӧ���ɿ�ȩ��֬��д���÷�Ӧ�Ļ�ѧ����ʽ ��
����5��E�ж�����ˮ�������F������Ԫ��״�ṹ��G��һ�־����߷��Ӳ��ϣ�д��F��G�Ľṹ��ʽ��F��
 G��
G�� ��
��
���� AΪ����ͬϵ���һ�ȴ�����5�֣�����A�ķ���ʽ��֪��AΪ ��C��D��E�ĺ˴Ź�������ͼ�У���ĸ����ֱ�Ϊ1��2��3������C�ķ���ʽ��֪��CΪCH3COCH3��C��HCN�����ӳɷ�Ӧ����DΪ��CH3��2COHCN������E�ķ���ʽ�Լ�������E�ֱ���������Na��������NaHCO3��Һ��Ӧ������ͬ�����£����ɵ�����������ͬ��֪��D�����������·���ˮ���EΪ��CH3��2COHCOOH���Ƚ�A��C�Ľṹ���B�ķ���ʽ��֪BΪ
��C��D��E�ĺ˴Ź�������ͼ�У���ĸ����ֱ�Ϊ1��2��3������C�ķ���ʽ��֪��CΪCH3COCH3��C��HCN�����ӳɷ�Ӧ����DΪ��CH3��2COHCN������E�ķ���ʽ�Լ�������E�ֱ���������Na��������NaHCO3��Һ��Ӧ������ͬ�����£����ɵ�����������ͬ��֪��D�����������·���ˮ���EΪ��CH3��2COHCOOH���Ƚ�A��C�Ľṹ���B�ķ���ʽ��֪BΪ ���ݴ˴��⣮
���ݴ˴��⣮
��� �⣺AΪ����ͬϵ���һ�ȴ�����5�֣�����A�ķ���ʽ��֪��AΪ ��C��D��E�ĺ˴Ź�������ͼ�У���ĸ����ֱ�Ϊ1��2��3������C�ķ���ʽ��֪��CΪCH3COCH3��C��HCN�����ӳɷ�Ӧ����DΪ��CH3��2COHCN������E�ķ���ʽ�Լ�������E�ֱ���������Na��������NaHCO3��Һ��Ӧ������ͬ�����£����ɵ�����������ͬ��֪��D�����������·���ˮ���EΪ��CH3��2COHCOOH���Ƚ�A��C�Ľṹ���B�ķ���ʽ��֪BΪ
��C��D��E�ĺ˴Ź�������ͼ�У���ĸ����ֱ�Ϊ1��2��3������C�ķ���ʽ��֪��CΪCH3COCH3��C��HCN�����ӳɷ�Ӧ����DΪ��CH3��2COHCN������E�ķ���ʽ�Լ�������E�ֱ���������Na��������NaHCO3��Һ��Ӧ������ͬ�����£����ɵ�����������ͬ��֪��D�����������·���ˮ���EΪ��CH3��2COHCOOH���Ƚ�A��C�Ľṹ���B�ķ���ʽ��֪BΪ ��
��
��1��CΪCH3COCH3��C���������������ŵ��������ʻ����ʴ�Ϊ���ʻ���
��2����������ķ�����֪��C��D�ķ�Ӧ�����Ǽӳɷ�Ӧ���ʴ�Ϊ���ӳɷ�Ӧ��
��3��AΪ ��A�ĺ��б�����ͬ���칹�壨��A�⣩Ϊ����������һ������Ϊ-CH2CH2CH3����������������Ϊ-CH3��-CH2CH3�����ڼ�����֣�����������-CH3�����ݶ�����һ��ԭ���֪��3�ֽṹ�����Թ���7�֣�
��A�ĺ��б�����ͬ���칹�壨��A�⣩Ϊ����������һ������Ϊ-CH2CH2CH3����������������Ϊ-CH3��-CH2CH3�����ڼ�����֣�����������-CH3�����ݶ�����һ��ԭ���֪��3�ֽṹ�����Թ���7�֣�
�ʴ�Ϊ�� ��7��
��7��
��4����ȩ�� ����һ����Ҫ�Ļ���ԭ�ϣ���ͬ��ȩ�뱽�ӷ�Ӧ���ɷ�ȩ��֬����Ҳ���뱽�ӷ�Ӧ���ɿ�ȩ��֬���÷�Ӧ�Ļ�ѧ����ʽΪ
����һ����Ҫ�Ļ���ԭ�ϣ���ͬ��ȩ�뱽�ӷ�Ӧ���ɷ�ȩ��֬����Ҳ���뱽�ӷ�Ӧ���ɿ�ȩ��֬���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��5��EΪ��CH3��2COHCOOH��E�ж�����ˮ�������F������Ԫ��״�ṹ��G��һ�־����߷��Ӳ��ϣ���FΪ ��GΪ
��GΪ ��
��
�ʴ�Ϊ�� ��
�� ��
��
���� ���⿼���л�����ƶϣ���Ŀ�Ѷ��еȣ�����Ĺؼ�����A���ƶϣ�����������Ϣ������ʵĹ����ŵı仯�����ƶϣ�ע����չ����ŵ����ʣ�

 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�| A�� | CO2�ĵ���ʽ�� | |
| B�� | Clԭ�ӵĽṹʾ��ͼ��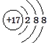 | |
| C�� | ������Ϊ53��������Ϊ78�ĵ�ԭ�ӵĺ��ط��ţ�${\;}_{53}^{131}$I | |
| D�� | 2��3-����-2-��ϩ�Ľṹ��ʽ��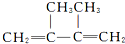 |
| A�� | �ñ����������Һ���������� | |
| B�� | ����ʯ�Ҹ��ﰱ�� | |
| C�� | �����Ƶ�����ʢװŨ���� | |
| D�� | �ñ���̼������Һ�ռ�ʵ������ȡ���������� |

��1���ϳɼ״��ķ�Ӧ���������������仯��ͼ1��ʾ��д���ϳɼ״����Ȼ�ѧ����ʽCO��g��+2H2��g��?CH3OH��g����H=-��b-a��kJ/mol��
��2��ʵ������lL�ܱ������н���ģ��ϳ�ʵ�飮��1molCO��2molH2ͨ�������У��ֱ������300���500�淴Ӧ��ÿ��һ��ʱ���������м״���Ũ�����£����������ݵ�λ��mol•L-l��
| �¶�\ʱ�� | 10min | 20min | 30min | 40min | 50min | 60min |
| 300�� | 0.40 | 0.60 | 0.75 | 0.84 | 0.90 | 0.90 |
| 500�� | 0.60 | 0.75 | 0.78 | 0.80 | 0.80 | 0.80 |
��500��ʱƽ�ⳣ��K����ֵΪ25��
��300��ʱ�����������ݻ�ѹ����ԭ����$\frac{1}{2}$���������������������£���ƽ����ϵ������Ӱ����cd��ѡ���ţ���
a��c��H2����С
b������Ӧ���ʼӿ죬�淴Ӧ���ʼ���
c��CH3OH�����ʵ�������
d������ƽ��ʱ$\frac{c��{H}_{2}��}{c��C{H}_{3}OH��}$��С
��3����ͼ2�Ǽ״�ȼ�ϵ�ع�����ʾ��ͼ������A��B��D��Ϊʯī�缫��CΪͭ�缫������һ��ʱ��Ͽ�K����ʱA��B�����ϲ��������������ͬ��
�ټ��и����ĵ缫��ӦʽΪCH3OH-6e-+8OH-=CO32-+6H2O��
������A�������������ڱ�״���µ����Ϊ2.24l��
�۷�Ӧ������Ҫʹ��װ���н���������ǡ����ȫ��������Ҫ300mL5.0mol•L-lNaOH ��Һ��
| Ԫ�ر�� | �� | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶/nm | 0.037 | 0.074 | 0.082 | 0.099 | 0.102 | 0.143 | 0.152 | 0.186 |
| ����ϼۻ� ��ͻ��ϼ� | +1 | -2 | +3 | -1 | -2 | +3 | +1 | +1 |
| A�� | Ԫ�آڢ��γɵĻ������������ | |
| B�� | Ԫ�آ���̬�⻯��ķе�С��Ԫ�آ���̬�⻯��ķе� | |
| C�� | Ԫ�آݶ�Ӧ�����Ӱ뾶����Ԫ�آ߶�Ӧ�����Ӱ뾶 | |
| D�� | Ԫ�آܵ�����������ˮ�����Ԫ�آݵ�����������ˮ��������ǿ |
| A�� | ����ܵı�����Ϳ���ᣬ��Ҫ��Ϊ������ | |
| B�� | ���ս������Ϲޣ�ֻ��Ϊ�˼��ٻ�����Ⱦ | |
| C�� | ���ĵ����Ա�ͭ�ã����Գ����������߶�����ͭ | |
| D�� | ����ֿ���ʴ�Ժã���������ҽ����е�����ߵ� |
| A�� | ̼������ȷֽ� | B�� | ����ȼ�� | ||
| C�� | ������������������Ӧ | D�� | �������ᷴӦ |
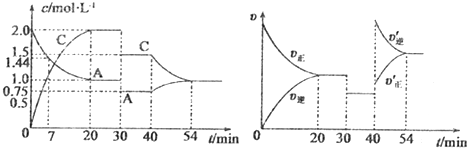

 ��
�� ����дһ����
����дһ����