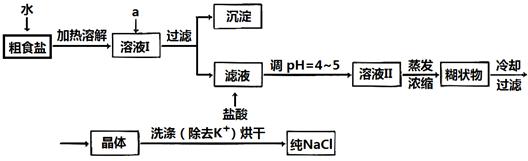
��Ŀ����
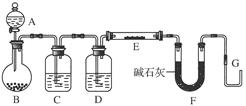
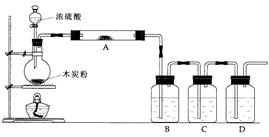
(8��)ij����С������H2��ԭ��ɫ��WO3��ĩ���ⶨW�����ԭ����������ͼ�Dzⶨװ�õ�ʾ��ͼ��A�е��Լ������ᡣ
��ش��������⣺
(1)������װ����Լ���B________��C________��D________��
(2)���Ӻ�װ�ú�Ӧ����__________________________________________________���䷽����______________________________________________________________________________________________________________________________________��
(3)�����ȷ�Ӧ��E���͡���Aƿ��εμ�Һ�塱����������Ӧ���Ƚ��е���
________________________________________________________________________��
��������֮�仹Ӧ���еIJ�����___________________________________________
________________________________________________________________________��
(4)��Ӧ������G���ݳ���������___________________________________________��
�䴦��������___________________________________________________________
________________________________________________________________________��
(5)��ʵ���в�����������ݣ�
�ٿ�E�ܵ�����a
��E�ܺ�WO3��������b
�۷�Ӧ��E�ܺ�W�۵�������c(��ȴ�����³���)
�ܷ�ӦǰF�ܼ���ʢ���������d
�ݷ�Ӧ��F�ܼ���ʢ���������e
���������ݿ����г�����W�����ԭ��������������ͬ����ʽ(��W�⣬�����漰��Ԫ�ص����ԭ��������Ϊ��֪)��
����ʽ1��Ar(W)��________������ʽ2��Ar(W)��________��

��ش��������⣺
(1)������װ����Լ���B________��C________��D________��
(2)���Ӻ�װ�ú�Ӧ����__________________________________________________���䷽����______________________________________________________________________________________________________________________________________��
(3)�����ȷ�Ӧ��E���͡���Aƿ��εμ�Һ�塱����������Ӧ���Ƚ��е���
________________________________________________________________________��
��������֮�仹Ӧ���еIJ�����___________________________________________
________________________________________________________________________��
(4)��Ӧ������G���ݳ���������___________________________________________��
�䴦��������___________________________________________________________
________________________________________________________________________��
(5)��ʵ���в�����������ݣ�
�ٿ�E�ܵ�����a
��E�ܺ�WO3��������b
�۷�Ӧ��E�ܺ�W�۵�������c(��ȴ�����³���)
�ܷ�ӦǰF�ܼ���ʢ���������d
�ݷ�Ӧ��F�ܼ���ʢ���������e
���������ݿ����г�����W�����ԭ��������������ͬ����ʽ(��W�⣬�����漰��Ԫ�ص����ԭ��������Ϊ��֪)��
����ʽ1��Ar(W)��________������ʽ2��Ar(W)��________��
(1)п��'ˮ'Ũ����
(2)���������'��G��ܽ�û��ʢ��ˮ���ձ��У�������ƿB���۲�G��ܿڣ����������ݳ�������ȴ��������γ�һ���ȶ�ˮ����˵��װ�õ�����������
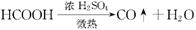
(3)��Aƿ��εμ�Һ��'����H2�Ĵ���
(4)����'��G�ܳ��ڴ���ȼ'(5)��
(2)���������'��G��ܽ�û��ʢ��ˮ���ձ��У�������ƿB���۲�G��ܿڣ����������ݳ�������ȴ��������γ�һ���ȶ�ˮ����˵��װ�õ�����������
(3)��Aƿ��εμ�Һ��'����H2�Ĵ���
(4)����'��G�ܳ��ڴ���ȼ'(5)��
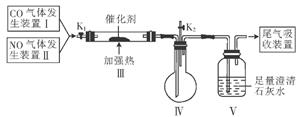
(1)�������ʵ��Ŀ�ģ�����ȷ��BΪ��ȡH2�ķ���װ�á�Ӧ�Ż��ý���Zn����C��D�dz���װ�÷ֱ��H2�е�HCl�����ˮ������
(2)����ʵ��ǰһ�����װ�õ������ԣ�����Ҫע����������һ���ȷ�����壬�ڶ����Է������ı�ѹǿ��������˵����Ӧ����
(3)H2�ڵ�ȼ������ǰһ��Ҫ�鴿��β��ҲӦ����ȼ�յ���
(2)����ʵ��ǰһ�����װ�õ������ԣ�����Ҫע����������һ���ȷ�����壬�ڶ����Է������ı�ѹǿ��������˵����Ӧ����
(3)H2�ڵ�ȼ������ǰһ��Ҫ�鴿��β��ҲӦ����ȼ�յ���

��ϰ��ϵ�д�
�����Ŀ
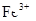
 ��֪��
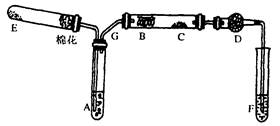
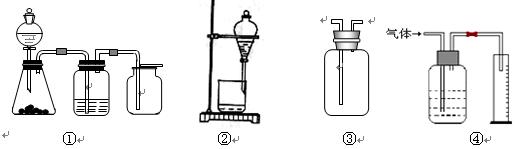
��֪�� ��ʵ��������ͼ��ʾ��װ��.����ȡһ����̼��ѡ�õ�װ��Ϊ________________(����ĸ����
��ʵ��������ͼ��ʾ��װ��.����ȡһ����̼��ѡ�õ�װ��Ϊ________________(����ĸ����
 ������ƽ��ҩ�ס����������___ _____�����������ƣ���
������ƽ��ҩ�ס����������___ _____�����������ƣ���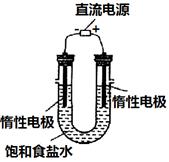
 MnCl2+Cl2��+2H2O���ݴˣ���������������װ����ѡ���Ʊ����ռ�H2��װ��_______(�����)���Ʊ����ռ��������Cl2��װ��_________������ţ���
MnCl2+Cl2��+2H2O���ݴˣ���������������װ����ѡ���Ʊ����ռ�H2��װ��_______(�����)���Ʊ����ռ��������Cl2��װ��_________������ţ���