��Ŀ����
����Ŀ������˵����ȷ����(����)
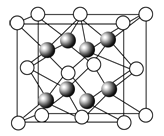
�ٱ�״���£�6.02��1023��������ռ�����Լ��22.4 L
��0.5 mol H2��ռ���Ϊ11.2 L
�۱�״���£�1 mol H2O�����Ϊ22.4 L
�ܱ�״���£�28 g CO��N2�Ļ����������ԼΪ22.4 L
�ݸ������������Ħ�������ԼΪ22.4 L��mol��1
�ޱ�״���£������ͬ������ķ�������ͬ
A. �٢ۢ�B. �ܢ�C. �ڢܢ�D. �٢ܢ�
���𰸡�B
��������
�ٸ����ʲ�һ��Ϊ���壬��Ϊ����ʱ����״���£�6.02��1023��������ռ�����Լ��![]() mol��22.4L/mol=22.4L���ʴ���
mol��22.4L/mol=22.4L���ʴ���
�ڲ�һ���ڱ�״���£���Vmδ֪����������V=nVm���㣬�ʴ���
�۱�״���£�ˮ�������壬���ܸ���V=nVm���㣬�ʴ���
��CO��N2��Ħ��������Ϊ28g/mol����״���£�28g CO��N2�Ļ����������ԼΪ![]() ��22.4L/mol=22.4L������ȷ��
��22.4L/mol=22.4L������ȷ��
������Ħ��������¶ȡ�ѹǿ�йأ�״̬δ֪������ȷ�������Ħ���������״���£��������������Ħ�������ԼΪ22.4Lmol-1���ʴ���
�ޱ�״���£������ͬ�����壬���ʵ�����ͬ����n=![]() ��֪����������ͬ������ȷ��
��֪����������ͬ������ȷ��
��ѡB��

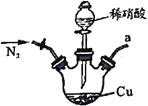
����Ŀ���������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�á�
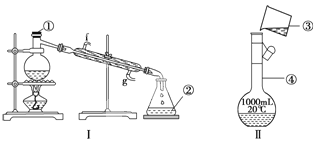
��1��д���������������ƣ���__________����__________��
��2��������װ�����������Ȼ�̼�;ƾ��Ļ�����ȱ�ٵ�������______________��������������������е�ʵ�����������Ϊ____________��
��3����Na2CO3��10H2O���壬����0.2 mol��L��1��Na2CO3��Һ480 mL��
��Ӧ��ȡNa2CO3��10H2O�����������__________��
������������������ƽ���ձ�����������ҩ�ס���Ͳ�⣬����Ҫ__________(����������)��
���������в�����������Һ��Ũ�Ȳ�����Ӱ�죬�������Ҫ��
A��Na2CO3��10H2O����ʧȥ�˲��ֽᾧˮ |
B�����������������ij���������������(ʹ������) |
C������̼���ƾ���ʱ������������ |
D������ƿδ������ʹ�� |
��������������ҺŨ��ƫ�ߵ���___________(����ĸ)
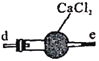
��װ������ijͬѧת����Һ��ʾ��ͼ��ͼ�еĴ�����__________________