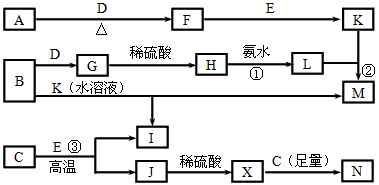
ΧβΡΩΡΎ»ί
AΓΪNΨυΈΣ÷–―ßΜ·―ß÷–ΒΡ≥ΘΦϊΈο÷ Θ§Τδ÷–A «»’≥Θ…ζΜν÷–≤ΜΩ…»±…ΌΒΡΈο÷ Θ§≥ΘΈ¬œ¬BΓΔEΓΔFΈΣΤχΧεΘ§GΈΣΈό…Ϊ“ΚΧεΘ§’β–©Έο÷ ‘Ύ“ΜΕ®ΧθΦΰœ¬¥φ‘Ύ»γœ¬ΉΣΜ·ΙΊœΒΘ§Τδ÷–”––©Ζ¥”ΠΈοΜρ…ζ≥…Έο“―Ψ≠¬‘»ΞΓΘΜΊ¥πœ¬Ν–Έ ΧβΘΚ?(1)BΚΆNΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΘ?
(2)»τ“‘MΈΣΒγΦΪΒγΫβAΒΡΥ°»ή“Κ ±Θ§―τΦΪ…œΒΡΒγΦΪΖ¥”Π ΫΈΣΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΘ§“θΦΪ…œΒΡΒγΦΪΖ¥”Π ΫΈΣΓΓΓΘ?

(3)D”κGΖ¥”Π…ζ≥…1.12 L±ξΉΦΉ¥Ωωœ¬ΒΡF ±Θ§ΉΣ“ΤΒΡΒγΉ” ΐΈΣ?ΓΓΓΓΓΓΓΓ?ΓΘ?
(4)G”κMΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΘ?
(5)IΩ…”Ο”ΎΨΜΜ·Υ°Θ§Τδ‘≠“ρ «(”ΟάκΉ”ΖΫ≥Χ Ϋ±μ Ψ)ΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΘ?
(6)»τ“‘Εη–‘ΒγΦΪΒγΫβAΒΡΥ°»ή“Κ ±Θ§Ά®Βγ“ΜΕΈ ±ΦδΚσΘ§―τΦΪ…œ…ζ≥…448 mLΒΡΤχΧε(±ξΉΦΉ¥Ωω)Θ§¥Υ ±»ή“ΚΒΡΧεΜΐΈΣ400 mLΘ§‘ρ»ή“ΚΒΡpHΈΣ?ΓΓΓΓΓΓΓΓ?ΓΘ?
(1)Cl2 + 2OH-ΘΫCl- + ClO-+ H2O?
(2)Fe - 2e-ΘΫ Fe2+,2H+ + 2e-ΘΫH2Γϋ?
(3)6.02ΓΝ1022??
(4)4H2O(g) + 3Fe ![]() Fe3O4 + 4H2
Fe3O4 + 4H2
(5)Fe3+ + 3H2O ΘΫ Fe(OH)3(ΫΚΧε)+ 3H+?
(6)13
ΫβΈωΘΚ”…ΩρΆΦΆΤ÷ΣAΈΣNaCl,BΈΣCl2,CΈΣNa,DΈΣNa2O2,‘ρGΈΣH2OΘ§EΈΣH2Θ§JΈΣHClΘ§MΈΣ±δΦέΫπ τ‘ρΈΣFe,IΈΣFeCl3,KΈΣFeCl2,FΈΣO2Θ§HΈΣFe3O4ΓΘ?
(1)B”κNΦ¥Cl2ΚΆNaOHΖ¥”ΠΓΘ?
(2)“‘ΧζΉςΒγΦΪΒγΫβNaCl»ή“ΚΘ§―τΦΪFe - 2e-ΘΫ Fe2+,“θΦΪ2H++2e-ΘΫH2ΓϋΓΘ?
(3)Na2O2”κH2OΖ¥”Π1 mol Na2O2Ζ¥”ΠΉΣ“ΤΒγΉ”1 mol,1.12 L O2ΈΣ0.05 mol–ηNa2O2 0.1 molΘ§Ι ΉΣ“ΤΒγΉ”ΈΣ0.1 molΓΘ?
(4)G”κMΦ¥Fe”κH2OΖ¥”ΠΘ§4H2O(g) + 3Fe ![]() Fe3O4 + 4H2ΓΘ?
Fe3O4 + 4H2ΓΘ?
(5)Fe3+Υ°Ϋβ…ζ≥…ΒΡFe(OH)3ΨΜΥ°ΓΘ?
(6)―τΦΪΖ¥”ΠΈΣΘΚ2Cl--2e-ΘΫCl2Γϋ,448 mL Cl2ΈΣ0.02 mol,‘ρΉΣ“ΤΒγΉ”0.04 mol,Νμ“ΜΒγΦΪ…œ”–0.04 mol H+Ζ¥”ΠΘ§Ά§ ±≤ζ…ζ0.04 mol OH-Θ§‘ρc(OH-)=![]() =0.1 molΓΛL-1,c(H+)=1ΓΝ10-13molΓΛL-1,pH=13ΓΘ
=0.1 molΓΛL-1,c(H+)=1ΓΝ10-13molΓΛL-1,pH=13ΓΘ

| |||||||||||||||||||||||
| |||||||||||||||||||||||||||