��Ŀ����
19��A��B��C��D��E��Ϊ������Ԫ�أ�A������Ԫ�������ԭ��������С�ģ�B��+2�������Ӻ�C��-1�������Ӷ�����ԭ�Ӿ�����ͬ�ĵ��Ӳ��Ų���D��C����һ���ڣ�����B�γ�BD2�����ӻ����E��CΪͬһ����Ԫ�أ�������������ˮ����Ϊǿ�ᣮ��ش��������⣺��1��Ԫ�ط��ţ�AH��CF
��2��E���ʵĵ���ʽ
 ��B��ԭ�������ڱ��е�λ���ǵ������ڢ�A��
��B��ԭ�������ڱ��е�λ���ǵ������ڢ�A����3��C��D��E�ֱ���A�γɵĻ����������ȶ�����HF���ѧʽ��
��4���õ���ʽ��ʾB��C�γɻ�����Ĺ��̣�

��5��C��A�γɵĻ�����ķе��D��A�γɵĻ�����ķе�HF��ԭ����HF����֮����������HCl����֮��Ϊ���»�����
���� A��B��C��D��E��Ϊ������Ԫ�أ�A������Ԫ�������ԭ��������С�ģ���AΪ��Ԫ�أ�B��+2�������Ӻ�C��-1�������Ӷ�����ԭ�Ӿ�����ͬ�ĵ��Ӳ��Ų�����BΪMgԪ�أ�CΪ��Ԫ�أ�D��C����һ���ڣ�����B�γ�BD2�����ӻ������DΪClԪ�أ�E��CΪͬһ����Ԫ�أ�������������ˮ����Ϊǿ�ᣬ��EΪNԪ�أ��ݴ˽��
��� �⣺A��B��C��D��E��Ϊ������Ԫ�أ�A������Ԫ�������ԭ��������С�ģ���AΪ��Ԫ�أ�B��+2�������Ӻ�C��-1�������Ӷ�����ԭ�Ӿ�����ͬ�ĵ��Ӳ��Ų�����BΪMgԪ�أ�CΪ��Ԫ�أ�D��C����һ���ڣ�����B�γ�BD2�����ӻ������DΪClԪ�أ�E��CΪͬһ����Ԫ�أ�������������ˮ����Ϊǿ�ᣬ��EΪNԪ�أ�
��1��������������֪��AΪHԪ�أ�CΪFԪ�أ��ʴ�Ϊ��H��F��
��2��E����ΪN2������ʽΪ ��BΪMgԭ�ӣ�ԭ�������ڱ��е�λ���ǵ������ڢ�A�壬
��BΪMgԭ�ӣ�ԭ�������ڱ��е�λ���ǵ������ڢ�A�壬
�ʴ�Ϊ�� ���������ڢ�A�壻
���������ڢ�A�壻
��3��C��D��E�ֱ���A�γɵĻ�����ֱ�ΪHF��HCl��NH3������FԪ�طǽ�������ǿ����HF���ȶ����ʴ�Ϊ��HF��
��4��B��C�γɻ�����ΪMgF2���õ���ʽ��ʾ���γɹ���Ϊ�� ��
��
�ʴ�Ϊ�� ��
��
��5��C��A�γɵĻ�����ΪHF��D��A�γɵĻ�����ΪHCl������HF����֮����������HCl����֮��Ϊ���»�������HF�е����HCl��
�ʴ�Ϊ��HF��HF����֮����������HCl����֮��Ϊ���»�����
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��ƶ�Ԫ���ǽ���ؼ���ע���õ���ʽ��ʾ��ѧ�������ʵ��γɣ�

| A�� | NaOH | B�� | AlCl3 | C�� | K2S | D�� | Cl2 |
| A�� | ��ϩ������8���Ҽ���1���м� | |
| B�� | ��ϩ������3��̼ԭ�Ӷ���sp3�ӻ� | |
| C�� | ��ϩ������ֻ���ڼ��Լ� | |
| D�� | ��ϩ������3��̼ԭ����ͬһֱ���� |
| A�� | Na2S | B�� | CaCl2 | C�� | Na2O2 | D�� | H2O2 |
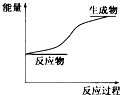
| A�� | �������������С�ڷ�Ӧ��������� | |
| B�� | �Ͽ���ѧ�������յ�����С���γɻ�ѧ�����ų������� | |
| C�� | �÷�Ӧ��������ͼ���кͷ�Ӧ | |
| D�� | �÷�Ӧ��������ʽ������ת�����˻�ѧ�� |
 �����£�ijNa2CO3��Һ�к���̼��ֺ��������ʵ�������������Һ��pH���Ͷ������仯��ͼ������˵����ȷ���ǣ�������
�����£�ijNa2CO3��Һ�к���̼��ֺ��������ʵ�������������Һ��pH���Ͷ������仯��ͼ������˵����ȷ���ǣ�������| A�� | �ڵ��з�̪��Na2CO3��Һ����μ���������Һǡ�ñ�Ϊ��ɫ���õ���Һ | |
| B�� | ��CO2����ų��ķ�Χ��pH��5 | |
| C�� | HCO3-�ĵ���ƽ�ⳣ��Ϊ1��10-4 | |
| D�� | pH=11��Na2CO3��Һϡ�͵����Ϊԭ����100����pH=9 |
| A�� | CH4 | B�� | NH3 | C�� | H2O | D�� | CO2 |
 ��ͼ����a��ʾ���ȷ�Ӧ X��g��+Y��g��?Z��g��+M��g��+N��s�����й�����X��ת������ʱ��仯�Ĺ�ϵ����Ҫ�ı���ʼ������ʹ��Ӧ���̰�b���߽��У��ɲ�ȡ�Ĵ�ʩ�ǣ�������
��ͼ����a��ʾ���ȷ�Ӧ X��g��+Y��g��?Z��g��+M��g��+N��s�����й�����X��ת������ʱ��仯�Ĺ�ϵ����Ҫ�ı���ʼ������ʹ��Ӧ���̰�b���߽��У��ɲ�ȡ�Ĵ�ʩ�ǣ�������| A�� | �Ӵ��� | B�� | ������� | C�� | �����¶� | D�� | �Ӵ�X��Ͷ���� |

 ��д���ٵ���̬�⻯����ӵĵ���ʽ
��д���ٵ���̬�⻯����ӵĵ���ʽ ��
��