��Ŀ����
�ڡ�̽��ˮ�ķ��ڡ�ʵ���У�ijʵ��С��۲쵽ͼ1��ͼ��ͼ����ʾ�������
��1������Ϊͼ______��ˮ����ʱ���龰��ѡ����ң���
��2��ʵ������з��֣��ӿ�ʼ��ˮ���ȵ�ˮ��ʼ���ڵ�ʱ�������Ϊ�����̼���ʱ�䣬�������������Ч�IJ������飺
��______��
��______��
��3��̽����������λͬѧ�ֱ������ˮ���¶Ⱥ�ʱ���ϵ��������ͼ2����������ȷ��ӳ��̽��ˮ�ķ��ڡ�ʵ��������¶���ʱ��仯��ϵ���ǣ�______��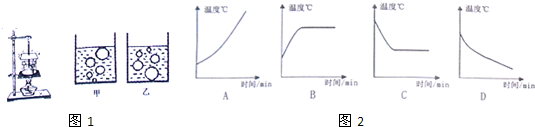
��2��Һ��Ӽ��ȵ�������Ҫ��ʱ��ij�����ܶ������йأ�ˮ���Ķ��١�ˮ���µĸߵ͡������Ĵ�С�����þƾ��Ƶ����滹��������ȵȵȣ����ִӿ�ʼ���ȵ����ڵ����ʱ���������Ϊ������ʵ���ʱ�䣬���Բ�ȡ�Ĵ�ʩ�ǣ���Сˮ�����������ˮ�ij��µȣ�
��3��ˮ�ڷ���ǰ�����¶����ߣ�����ʱ�����¶ȱ��ֲ��䣬����ͼB��ˮ�ķ���ͼ��
�ʴ�Ϊ����1���ף���2���ټ���ˮ�������������ˮ�ij��£���3��B��
��������1�����շ���ʱ�ͷ���ǰ��������ǰ�����������������������С����ˮ����ʧ������ʱ���д������ݲ������������������������������������ѣ�
��2��Һ��Ӽ��ȵ�������Ҫ��ʱ��ij�����ܶ������йأ�ˮ���Ķ��١�ˮ�µĸߵ͡������Ĵ�С�����þƾ��Ƶ����滹��������ȵȵȣ�
��3������ˮ����ǰ�ͷ���ʱ�¶ȵı仯��������жϣ�����ǰ�����¶����ߣ�����ʱ�����¶ȱ��ֲ��䣮
������������̽��ˮ�ķ���ʵ�飬�����˷���ǰ����ʱ���������𣬹ؼ���ͨ�����ݵı仯�����жϣ�������Ӱ��ˮ����ʱ������ؼ�����ͼ��Ļ�������һ���ܵ��͵�ʵ�飮

 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д���1��С���۲쵽ˮ����ǰ�ͷ���ʱ��ˮ����������������ͼ1��ʾ����ͼ��________�Ƿ���ǰ�����(��ס����ҡ�)��
 | |||
 | |||
��2���±���ʵ��������¼�����ݣ�
| ʱ�䣨min�� | �� | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | �� |
| �¶ȣ��棩 | �� | 95 | 96 | 97 | 98 | 98 | 98 | 98 | 96 | 98 | 98 | �� |
���ɱ������ݵó��Ľ����ǣ�__________________ ��
��3��ͬʱ��С��Ҳ���˸�ʵ�顣���Ǹ��ݸ��Ե����ݣ���ͬһ����ֽ�ϻ�������ͼ2��ʾ��ͼ�ߣ�����ʵ�߱�ʾС����ͼ�ߣ����߱�ʾС����ͼ�ߣ�ˮƽ����ͼ���غϡ�������������ȫ��ͬ���������������˵IJ������淶ȷ������ͼ�߷�����
����Ϊ�����ͬѧͼ�߲������Ҫԭ������ǣ�__________________________ ��
��4��С������������ͬѧ��õķе���Լ����������1�棬����Ϊ������ͬѧ��ʵ�����¶ȼƵIJ������������ձ�����ɵģ�����Ϊ�����뷨��ȷ�� ������ȷ��������
���ڡ�̽��ˮ�ķ����ص㡱��У�С����װ��ʵ��װ�ã���������ʵ�飬���������С���������ʵ���е�һЩ���ݣ�
��1��С���۲쵽ˮ����ǰ�ͷ���ʱ��ˮ����������������ͼ1��ʾ����ͼ��________�Ƿ���ǰ�����(��ס����ҡ�)��
 | |||
 | |||
��2���±���ʵ��������¼�����ݣ�
| ʱ�䣨min�� | �� | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | �� |
| �¶ȣ��棩 | �� | 95 | 96 | 97 | 98 | 98 | 98 | 98 | 96 | 98 | 98 | �� |
���ɱ������ݵó��Ľ����ǣ�__________________ ��
��3��ͬʱ��С��Ҳ���˸�ʵ�顣���Ǹ��ݸ��Ե����ݣ���ͬһ����ֽ�ϻ�������ͼ2��ʾ��ͼ�ߣ�����ʵ�߱�ʾС����ͼ�ߣ����߱�ʾС����ͼ�ߣ�ˮƽ����ͼ���غϡ�������������ȫ��ͬ���������������˵IJ������淶ȷ������ͼ�߷�����
����Ϊ�����ͬѧͼ�߲������Ҫԭ������ǣ�__________________________ ��
��4��С������������ͬѧ��õķе���Լ����������1�棬����Ϊ������ͬѧ��ʵ�����¶ȼƵIJ������������ձ�����ɵģ�����Ϊ�����뷨��ȷ�� ������ȷ��������
���ڡ�̽��ˮ�ķ����ص㡱��У�С����װ��ʵ��װ�ã���������ʵ�飬���������С���������ʵ���е�һЩ���ݣ�
��1��С���۲쵽ˮ����ǰ�ͷ���ʱ��ˮ����������������ͼ1��ʾ����ͼ��________�Ƿ���ǰ�����(��ס����ҡ�)��
 |
|||
 |
|||
��2���±���ʵ��������¼�����ݣ�
|
ʱ�䣨min�� |
�� |
0 |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
�� |
|
�¶ȣ��棩 |
�� |
95 |
96 |
97 |
98 |
98 |
98 |
98 |
96 |
98 |
98 |
�� |
�ٴӼ�¼���ݿ����ڵ�_________minʱ�������Ǵ���ģ��¶�Ӧ��Ϊ_________��
���ɱ������ݵó��Ľ����ǣ�__________________ ��
��3��ͬʱ��С��Ҳ���˸�ʵ�顣���Ǹ��ݸ��Ե����ݣ���ͬһ����ֽ�ϻ�������ͼ2��ʾ��ͼ�ߣ�����ʵ�߱�ʾС����ͼ�ߣ����߱�ʾС����ͼ�ߣ�ˮƽ����ͼ���غϡ�������������ȫ��ͬ���������������˵IJ������淶ȷ������ͼ�߷�����
����Ϊ�����ͬѧͼ�߲������Ҫԭ������ǣ�__________________________ ��
��4��С������������ͬѧ��õķе���Լ����������1�棬����Ϊ������ͬѧ��ʵ�����¶ȼƵIJ������������ձ�����ɵģ�����Ϊ�����뷨��ȷ�� ������ȷ��������

