��Ŀ����
��֪������y1=a��x-2��2-4��a��0�������㣨0��-3��������ΪM����������y1����ƽ��b����λ��ʹƽ�ƺ�õ���������y2��������ԭ�㣬������y2�Ķ���ΪA����x�����һ������ΪB��

��1����a��ֵ��
��2����b=________����������y2�ĺ�������ʽ��________��
��3���ٵ�P��y����һ�㣬��|PA-PB|��ֵ���ʱ�����P�����ꣻ
�ڵ�E��x����һ�㣬��������y2���Ƿ���ڵ�F��ʹO��ԭ�㣩��M��E��F�ĵ㹹����OMΪһ�ߵ�ƽ���ı��Σ������ڣ��������F�����ꣻ�������ڣ���˵�����ɣ�
�⣺��1��������y1=a��x-2��2-4��a��0�������㣨0��-3�����ɵã�
-3=a��0-2��2-4��
��ã�a=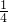 ��
��
��2���߾�����0��-3����������y1����ƽ�ƣ�������0��0���õ�������y2��
������ƽ����3����λ����b=3��
��������y2��y2=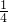 ��x-2��2-4+3=
��x-2��2-4+3=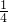 ��x-2��2-1��
��x-2��2-1��
 ��3���١�|PA-PB|��AB���ҵ��ҽ���P��A��B����ʱȡ�Ⱥţ�
��3���١�|PA-PB|��AB���ҵ��ҽ���P��A��B����ʱȡ�Ⱥţ�
��|PA-PB|��ֵ���ʱ��P��A��B���ߣ�
�ɣ�2���������߽���ʽ֪��A��2��-1����B��4��0������ֱ��AB�Ľ���ʽ��y=kx+b���У�
 ��
��
���
��ֱ��AB��y= x-2����P��0��-2����
x-2����P��0��-2����
����֪M��2��-4����������������ۣ�
��F��x���·�ʱ������OM��ƽ���ı��εıߣ���MF��x�ᣬ��F���������Ϊ-4����Ȼ��F��������������y2�ϣ����������������
��F��x���Ϸ�ʱ������ƽ���ı��������ĶԳ�ͼ�Σ�����F���������Ϊ4��
��y2=4ʱ��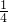 ��x-2��2-1=4����ã�x=2��2
��x-2��2-1=4����ã�x=2��2
��F��2-2 ��4����2+2
��4����2+2 ��4����
��4����
���ϣ����ڷ���������F�㣬������Ϊ��2-2 ��4����2+2
��4����2+2 ��4����
��4����
��������1������0��-3������y1=a��x-2��2-4��a��0���У��������a��ֵ��
��2��������y1������0��-3��������ƽ�ƺ�ԭ�㼴�ɣ�0��0�����������������ƽ����3����λ�����ݡ��ϼ��¼�����ƽ�ƹ��ɼ��ɵó�y2�ĺ�������ʽ��
��3���ٵ�P��A��B���㲻��ͬһֱ����ʱ���ܹ��ɡ�PAB�������������߹�ϵ�������ѿ���|PA-PB|��AB����P��A��B���㹲��ʱ��|PA-PB|=AB����Ȼ��|PA-PB|��ֵ���ʱ��P��A��B���㹲�ߣ�����ֱ�����ֱ��AB�Ľ���ʽ����ֱ����y��Ľ��㼴Ϊ����������P�㣻
�ڵ�O��M�Ѿ�ȷ���˾������꣬��OM��ƽ���ı��εıߣ�����ֻ������һ��EF���ɣ����ڵ�E��x���ϣ���OM EF�����Կɷ�����������ۣ�
EF�����Կɷ�����������ۣ�
��F��x���·�����ʱMF����OBƽ�У���MFƽ����x�ᣬ��ʱM��F�������������ͬ�������⣨y2��y1����ƽ�����ã���֪����F��������y2�ϣ����������������
��F��x���·�������ƽ���ı��������ĶԳ�ͼ�Σ���ô��ʱM��F�������껥Ϊ�෴�����ɾݴ�ȷ����F�����꣮
������������Ҫ�����˶��κ�������ʽ��ȷ��������ͼ���ƽ�ƹ��ɡ����������߹�ϵ�����Լ�ƽ���ı��ε��ж�����Ҫ֪ʶ����3�����ѶȽϴ����ü���֪ʶ�ҳ������˼·�ǽ���Ĺؼ����������������ν�ϵ���Ҫ�ԣ�
-3=a��0-2��2-4��
��ã�a=
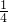 ��
����2���߾�����0��-3����������y1����ƽ�ƣ�������0��0���õ�������y2��
������ƽ����3����λ����b=3��
��������y2��y2=
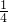 ��x-2��2-4+3=
��x-2��2-4+3=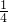 ��x-2��2-1��
��x-2��2-1�� ��3���١�|PA-PB|��AB���ҵ��ҽ���P��A��B����ʱȡ�Ⱥţ�
��3���١�|PA-PB|��AB���ҵ��ҽ���P��A��B����ʱȡ�Ⱥţ���|PA-PB|��ֵ���ʱ��P��A��B���ߣ�
�ɣ�2���������߽���ʽ֪��A��2��-1����B��4��0������ֱ��AB�Ľ���ʽ��y=kx+b���У�
 ��
�����

��ֱ��AB��y=
 x-2����P��0��-2����
x-2����P��0��-2��������֪M��2��-4����������������ۣ�
��F��x���·�ʱ������OM��ƽ���ı��εıߣ���MF��x�ᣬ��F���������Ϊ-4����Ȼ��F��������������y2�ϣ����������������
��F��x���Ϸ�ʱ������ƽ���ı��������ĶԳ�ͼ�Σ�����F���������Ϊ4��
��y2=4ʱ��
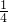 ��x-2��2-1=4����ã�x=2��2
��x-2��2-1=4����ã�x=2��2
��F��2-2
 ��4����2+2
��4����2+2 ��4����
��4�������ϣ����ڷ���������F�㣬������Ϊ��2-2
 ��4����2+2
��4����2+2 ��4����
��4������������1������0��-3������y1=a��x-2��2-4��a��0���У��������a��ֵ��
��2��������y1������0��-3��������ƽ�ƺ�ԭ�㼴�ɣ�0��0�����������������ƽ����3����λ�����ݡ��ϼ��¼�����ƽ�ƹ��ɼ��ɵó�y2�ĺ�������ʽ��
��3���ٵ�P��A��B���㲻��ͬһֱ����ʱ���ܹ��ɡ�PAB�������������߹�ϵ�������ѿ���|PA-PB|��AB����P��A��B���㹲��ʱ��|PA-PB|=AB����Ȼ��|PA-PB|��ֵ���ʱ��P��A��B���㹲�ߣ�����ֱ�����ֱ��AB�Ľ���ʽ����ֱ����y��Ľ��㼴Ϊ����������P�㣻
�ڵ�O��M�Ѿ�ȷ���˾������꣬��OM��ƽ���ı��εıߣ�����ֻ������һ��EF���ɣ����ڵ�E��x���ϣ���OM
 EF�����Կɷ�����������ۣ�
EF�����Կɷ�����������ۣ���F��x���·�����ʱMF����OBƽ�У���MFƽ����x�ᣬ��ʱM��F�������������ͬ�������⣨y2��y1����ƽ�����ã���֪����F��������y2�ϣ����������������
��F��x���·�������ƽ���ı��������ĶԳ�ͼ�Σ���ô��ʱM��F�������껥Ϊ�෴�����ɾݴ�ȷ����F�����꣮
������������Ҫ�����˶��κ�������ʽ��ȷ��������ͼ���ƽ�ƹ��ɡ����������߹�ϵ�����Լ�ƽ���ı��ε��ж�����Ҫ֪ʶ����3�����ѶȽϴ����ü���֪ʶ�ҳ������˼·�ǽ���Ĺؼ����������������ν�ϵ���Ҫ�ԣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 9����֪������y1=x2-2x+c�IJ���ͼ����ͼ��ʾ����ϵ��c��ȡֵ��Χ�ǣ�������
9����֪������y1=x2-2x+c�IJ���ͼ����ͼ��ʾ����ϵ��c��ȡֵ��Χ�ǣ������� ��ͼ����֪������y1=-2x2+2��ֱ��y2=2x+2����x��ȡһֵʱ��x��Ӧ�ĺ���ֵ�ֱ�Ϊy1��y2����y1��y2��ȡy1��y2�еĽ�Сֵ��ΪM����y1=y2����M=y1=y2�����磺��x=1ʱ��y1=0��y2=4��y1��y2����ʱM=0�������жϣ�
��ͼ����֪������y1=-2x2+2��ֱ��y2=2x+2����x��ȡһֵʱ��x��Ӧ�ĺ���ֵ�ֱ�Ϊy1��y2����y1��y2��ȡy1��y2�еĽ�Сֵ��ΪM����y1=y2����M=y1=y2�����磺��x=1ʱ��y1=0��y2=4��y1��y2����ʱM=0�������жϣ� ��2013•��ˮ��ģ����ͼ����֪������y1=-x2+1��ֱ��y2=-x+1����x��ȡһֵʱ��x��Ӧ�ĺ���ֵ�ֱ�Ϊy1��y2����y1��y2��ȡy1��y2�еĽ�Сֵ��ΪM1����y1=y2����M=y1=y2�����磺x=2ʱ��y1=-3��y2=-1��y1��y2��M=-3�������жϣ�
��2013•��ˮ��ģ����ͼ����֪������y1=-x2+1��ֱ��y2=-x+1����x��ȡһֵʱ��x��Ӧ�ĺ���ֵ�ֱ�Ϊy1��y2����y1��y2��ȡy1��y2�еĽ�Сֵ��ΪM1����y1=y2����M=y1=y2�����磺x=2ʱ��y1=-3��y2=-1��y1��y2��M=-3�������жϣ�