��Ŀ����
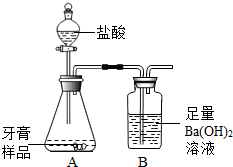
��ѧ��ȤС���ijƷ��������̼��ƺ�����������̽����������Ħ������Ҫ��̼��ơ�����������ɣ������ɷ���������ʱ���������ɣ���������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨB�����ɵ�BaCO3������������ȷ��̼��Ƶ�������������ش��������⣮��1��װ��A��������Ʒ������______�����������ƣ��У����������װ��______�����������ƣ��У�A�����ٷ���______�������֣�����Ӧ��д������һ����Ӧ�Ļ�ѧ����ʽ______��
��2�����ⶨֵ��ʵ��ֵС�����ܵ�ԭ����______��������ĸ��
a��װ��A��ˮ������HCl�Ƚ���װ��B�� b��װ��A��CO2δ��ȫ����װ��B
c��CO2�����ٶ�̫�쵼��Ba��OH��2δ��ȫ���� d������δ�μӹ���
��3��Ϊ�������ֲ�ȡ����һЩ��ʩ��
����A��B֮������һ��װ��______���ѧʽ����Һ��ϴ��ƿ������������______��
�ڽ�װ��B�еij������·�����һ���������ݣ��������______��
����װ��B�ĺ���������һ��װ��______����ҩƷ���ƻ�ѧʽ������ĸ���ܣ�Ŀ����______��
��4��ȷ��ȡ���ݸ�16.00g����Ʒ�����вⶨ�����BaCO3�����ֱ�Ϊ3.96g��3.92g��3.94g���������Ʒ��̼��Ƶ�����������
______����д��������̣���
��5��ijͬѧ�������������ⶨ�����и�Ԫ�ص�����������С��ͬѧ��Ϊ��ʹ�ų�ʵ�������Ͳ�����Ӱ�죬�����ⶨ�Ľ�����п���ƫС��������______��

���𰸡���������1��װ��A��������Ʒ��������ƿ�У�������Ӧ�÷��ڷ�Һ©���У�̼��ƺ������������ܹ������ᷴӦ�����Ծݴ���ɸ���Ľ��
��2��Ϊ�˱�֤ʵ������ȷ��Ҫ�����ܵ�ʹA�е��������B�У�ͬʱʹ����������ٶ�Ҫ�������μ�������ϡ������ʹ��Ӧ��ȫ�����Ծݴ˽�������ѡ����з�������ɽ��
��3��Ϊ��ֹHCl����Bװ�ã�����A��B֮������װ����������װ�ã���װ��B�еij������·�����һ���������ݣ������˽Ӵ������������߶�����̼������Ч�ʣ���װ��B�ĺ���������һ��װ�м�ʯ�һ������Ƶ�ҩƷ��װ�ã�������ֹ�����еĶ�����̼���룬���Ծݴ˽����⣻
��4������̼�ᱵ�������������̼�����������ݶ�����̼��������̼��Ƶ����������������������
��5����������к��������ܹ���ϡ���ᷴӦ�ĸƵĻ������ᵼ�²������ƫС�����Ծݴ˽����⣮
����⣺��1��װ��A��������Ʒ��������ƿ�У�������Ӧ�÷��ڷ�Һ©���У�̼��ƺ������������ܹ������ᷴӦ������װ��A�����ٷ���������Ӧ����Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2����Al��OH��3+3HCl�TAlCl3+3H2O��
��2��װ��A��ˮ������HCl�Ƚ���װ��B�У�����ɽ��ƫ��װ��A��CO2δ��ȫ����װ��B��CO2�����ٶ�̫�쵼��Ba��OH��2δ��ȫ���ա�����δ�μӹ�������ʹ������̼�������٣����²ⶨֵ��ʵ��ֵС������ȷ��ѡ��Ϊ��bcd
��3����Ϊ��ֹHCl����Bװ�ã�Ӧ����A��B֮������װ����������װ�ã�
�ڽ�װ��B�еij������·�����һ���������ݣ������˽Ӵ������������߶�����̼������Ч�ʣ�
����װ��B�ĺ���������һ��װ�м�ʯ�һ������Ƶ�ҩƷ��װ�ã�������ֹ�����еĶ�����̼���룻
��4�����̼�ᱵ������Ϊ��3.96g+3.92g+3.94g��÷3=3.94g
̼��Ƶ���������Ϊx
CaCO3��CO2��BaCO3��
100 197
16g?x 3.94g
 =
=
x=12.5%��
����Ʒ��̼��Ƶ���������Ϊ12.5%��
��5����ʵ������ͨ���ⶨ������̼����������ӵIJ���̼��Ƶ����������ģ���������к��������ĺ��иƵĻ���������ܼ������Իᵼ�²������ƫ�ͣ�
�ʴ�Ϊ����1����ƿ����Һ©����2��CaCO3+2HCl=CaCl2+H2O+CO2����Al��OH��3+3HCl=AlCl3+3H2O��
��2��bcd��
��3����AgNO3����ȥHCl���壻
�ڶ�����̼����Ч�ʣ�
�ۼ�ʯ�һ������Ƶȣ���ֹ�����е�CO2����װ��B��
��4��12.5%��
��5�������п��ܺ��иƵ����������
�����������������ε�������ʵ�鷽�����̵�̽�����������ʵĻ�ѧ���ʣ�Ҫ�������������Ҫ����ʵ����Ƶ�ÿһ�������е�ʵ��Ŀ�ģ���Ҫ��ǿ�����ȥ�������ӵķ������Ӷ�ͻ���ѵ㣮�йصļ���Ҫȷ�������㾭��������ʵ�����У�
��2��Ϊ�˱�֤ʵ������ȷ��Ҫ�����ܵ�ʹA�е��������B�У�ͬʱʹ����������ٶ�Ҫ�������μ�������ϡ������ʹ��Ӧ��ȫ�����Ծݴ˽�������ѡ����з�������ɽ��
��3��Ϊ��ֹHCl����Bװ�ã�����A��B֮������װ����������װ�ã���װ��B�еij������·�����һ���������ݣ������˽Ӵ������������߶�����̼������Ч�ʣ���װ��B�ĺ���������һ��װ�м�ʯ�һ������Ƶ�ҩƷ��װ�ã�������ֹ�����еĶ�����̼���룬���Ծݴ˽����⣻
��4������̼�ᱵ�������������̼�����������ݶ�����̼��������̼��Ƶ����������������������
��5����������к��������ܹ���ϡ���ᷴӦ�ĸƵĻ������ᵼ�²������ƫС�����Ծݴ˽����⣮
����⣺��1��װ��A��������Ʒ��������ƿ�У�������Ӧ�÷��ڷ�Һ©���У�̼��ƺ������������ܹ������ᷴӦ������װ��A�����ٷ���������Ӧ����Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2����Al��OH��3+3HCl�TAlCl3+3H2O��
��2��װ��A��ˮ������HCl�Ƚ���װ��B�У�����ɽ��ƫ��װ��A��CO2δ��ȫ����װ��B��CO2�����ٶ�̫�쵼��Ba��OH��2δ��ȫ���ա�����δ�μӹ�������ʹ������̼�������٣����²ⶨֵ��ʵ��ֵС������ȷ��ѡ��Ϊ��bcd
��3����Ϊ��ֹHCl����Bװ�ã�Ӧ����A��B֮������װ����������װ�ã�
�ڽ�װ��B�еij������·�����һ���������ݣ������˽Ӵ������������߶�����̼������Ч�ʣ�
����װ��B�ĺ���������һ��װ�м�ʯ�һ������Ƶ�ҩƷ��װ�ã�������ֹ�����еĶ�����̼���룻
��4�����̼�ᱵ������Ϊ��3.96g+3.92g+3.94g��÷3=3.94g
̼��Ƶ���������Ϊx
CaCO3��CO2��BaCO3��
100 197
16g?x 3.94g
 =
=
x=12.5%��
����Ʒ��̼��Ƶ���������Ϊ12.5%��
��5����ʵ������ͨ���ⶨ������̼����������ӵIJ���̼��Ƶ����������ģ���������к��������ĺ��иƵĻ���������ܼ������Իᵼ�²������ƫ�ͣ�
�ʴ�Ϊ����1����ƿ����Һ©����2��CaCO3+2HCl=CaCl2+H2O+CO2����Al��OH��3+3HCl=AlCl3+3H2O��
��2��bcd��
��3����AgNO3����ȥHCl���壻
�ڶ�����̼����Ч�ʣ�
�ۼ�ʯ�һ������Ƶȣ���ֹ�����е�CO2����װ��B��
��4��12.5%��
��5�������п��ܺ��иƵ����������
�����������������ε�������ʵ�鷽�����̵�̽�����������ʵĻ�ѧ���ʣ�Ҫ�������������Ҫ����ʵ����Ƶ�ÿһ�������е�ʵ��Ŀ�ģ���Ҫ��ǿ�����ȥ�������ӵķ������Ӷ�ͻ���ѵ㣮�йصļ���Ҫȷ�������㾭��������ʵ�����У�

��ϰ��ϵ�д�
 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�
�����Ŀ
 ��ѧ��ȤС���ijƷ��������̼��ƺ�����������̽����
��ѧ��ȤС���ijƷ��������̼��ƺ�����������̽���� ��ʵ��һ����װ�����۲�������ʣ�����������ҪΪ��ɫ��ĩ�����л��С�����ɫ������С����
��ʵ��һ����װ�����۲�������ʣ�����������ҪΪ��ɫ��ĩ�����л��С�����ɫ������С����
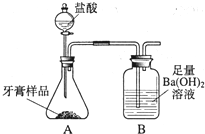 ��ѧ��ȤС���ijƷ��������̼��ƺ�����������̽����������Ħ������Ҫ��̼��ơ�����������ɣ������ɷ���������ʱ���������ɣ���������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨB�����ɵ�BaCO3������������ȷ��̼��Ƶ�������������ش��������⣮
��ѧ��ȤС���ijƷ��������̼��ƺ�����������̽����������Ħ������Ҫ��̼��ơ�����������ɣ������ɷ���������ʱ���������ɣ���������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨB�����ɵ�BaCO3������������ȷ��̼��Ƶ�������������ش��������⣮