��Ŀ����
�����ԭ�ӽṹ����ʶ����ֹ����
(1)��������Ϊԭ���ǡ������ٷֵ�ʵ�����塱����ķɭ��Ϊԭ���ǡ�Ƕ�����Ѹɵ�������������Щ�۵��_ _(���ȷ������)��¬ɪ�����Ц�����ɢ��ʵ�����Ϊԭ���ǡ�����ģ�͡�����ԭ�����ɴ�_ _��ɵ�ԭ�Ӻ˺ͺ�����ӹ��ɡ�
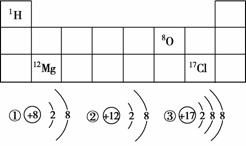
(2)��ͼ��Ԫ�����ڱ���һ����(���ֱ�ʾ��ӦԪ�ص�ԭ ������)�����в���Ԫ�ص�ԭ��(����)�ṹʾ��ͼ���£��������������ӵ���__(���������)��
������)�����в���Ԫ�ص�ԭ��(����)�ṹʾ��ͼ���£��������������ӵ���__(���������)��
ѡ�ñ���Ԫ����գ�A2B2�ͻ�����Ļ�ѧʽ��_ _����һ����λ����ɵ�һ����������_ _��ij�ͻ�� ������Mg��O��ɵĻ����д���仯ѧʽ_ _��
������Mg��O��ɵĻ����д���仯ѧʽ_ _��
���� ���� H2O2 Cl�� MgO

��ϰ��ϵ�д�
�����Ŀ
��һ��������a��b��c��d�������ʷ���һ�ܱ������У���һ�������·�Ӧһ��ʱ���÷�Ӧ������ʵ������� �¡�����˵������ȷ����( )
�¡�����˵������ȷ����( )
| ���� | a | b | c | d |
| ��Ӧǰ������(g) | 6.4 | 3.2 | 4.0 | 2.8 |
| ��Ӧ�������(g) | 5.2 | X | 7.2 | 2.8 |
A.a��c�Ƿ�Ӧ�� B��dһ���Ǵ���
C��X��2.0 g D���÷�Ӧ�ǻ��Ϸ�Ӧ

 ֱ�Ӽ��ȵ���__��
ֱ�Ӽ��ȵ���__��