��Ŀ����
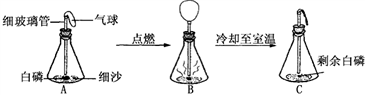
���ð���(��������������ͬ���Ż��Ϊ40��)̽�������غ㶨�ɵ�ʵ���У�����ͬѧ����Ƥ���ϵ�ϸ�������¶˷ŵ��ƾ��ƻ��������������Ⱥ�Ѹ������Ƥ������ƿ����������ȼ���ף�ʵ���������ͼ��ʾ����ش��������⣺

(1)ʵ��۲쵽A��C������Ĵ�С��ͬ��������֪ʶ���Ͳ����������ԭ����
��
(2)��C�ٴηŵ���ƽ�ϳ�������ƽ��Ȼƽ�⣬�ڴ˻�ѧ��Ӧ�У��Ӿ���ķ�Ӧ��������������ƽƽ���ԭ���� ��
77��(�����)д�����з�Ӧ�Ļ�ѧ����ʽ��
(1)���������г��ȼ��������������������������
(2)ˮ��ͨ�������·ֽ�������������������������
(3)��������ͭ��Һ��Ӧ������������������������
(4)����������Һ��ϡ���ᷴӦ����������������������
(1) S + O2 ��ȼ SO2
(2) 2H2O ͨ�� 2H2��+ O2 ��
(3) Fe + CuSO4 �� FeSO4 + Cu
(4) HCl + NaOH �� NaCl +H2O

��ϰ��ϵ�д�
 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
�����Ŀ