��Ŀ����
2006��ף��ҹ��䲼�µ���������ˮ���������ñ��а����������ݣ�
| ָ�� | �����Ŀ����λ | ��ֵ |
| ����ָ�� | �ܴ˾�Ⱥ��MPN/100ml�� | ���ü�� |
| �й���״ָ�� | ����ζ | �����ᡢ����ζ |
| ���ۿɼ��� | �� | |
| һ�㻯ѧָ�� | pH | ��С��6.5�Ҳ�����8.5 |
| ��Ӳ�ȣ���CaCO3�ƣ���mg/l�� | 450 | |
| �ܽ����ܹ��壨 mg/l�� | 1000 |
��1��������ˮ�����У���������Ȼˮ����ζ����________��
��2���������ȣ�ClO2����һ����ˮ�����ȷ����й㷺Ӧ�õĸ�Ч��ȫ����������������Cl2����ȣ��������Ȳ������и�ǿ��ɱ�����������Ҳ��������������DZ��Σ�����л��Ȼ��
�ٶ������ȣ�ClO2���У���Ԫ�صĻ��ϼ�Ϊ________��
���ڶ������ȵ��Ʊ������У�����������;����
����һ��2NaClO3+H2O2+H2SO4=2ClO2��+Na2SO4+O2��+2H2O
��������2NaClO3+4HCl=2ClO2��+Cl2��+2NaCl+2H2O
���������ַ����Ʊ��Ķ������ȣ����ʺ���������ˮ��������________�������һ��������������
����������ˮ������ʱ���������·�Ӧ��Cl2+H2O=HClO+HCl���������������������ˮ���ܶ���Ϊ1g/cm3������������������Ϊ1%��������Һ�����ʵ�����������Ӱ�����________������ĸ����
A��ϡ���ᡡ����B��̼������Һ����C������������Һ��������D���������Һ
��3��ˮ�ʼ���Ա�Ը���ָ����м���
�ټ��ˮ������Ӧ����________������ţ���
A����ɫʯ����Һ���������������� B����ɫ��̪��Һ��������C��pH��ֽ
����Ȼˮ��һ�㶼����Ca2+��Mg2+�����ӣ���Ca2+��Mg2+�ܺ�����������ˮӲ�ȱ�ʱ����Ϊ���ϸ�ˮ�ʼ���Ա�ⶨ1Lij��������ˮ�к���Ԫ��0.1g��þԪ��0.06g������24gþԪ���൱��40g��Ԫ�ػ����Ӳ�ȣ��жϸ�ˮ��________ ������ϡ������ϡ����ҹ�����ˮ���е���Ӳ�ȣ�
�⣺��1������̿���������ԣ�������ˮ�е�ɫ�غ���ζ�����Ա����Ϊ������̿��
��2��������Ԫ�صĻ��ͼ�Ϊx��x+��-2����2=0�����x=+4�����Ա����Ϊ��+4��
�ڷ������в���������Ҳ������������ˮ���������Ա����Ϊ����������
������������ˮ��Ӧ���ɵ����ʷ�Ӧ�ģ����ʺ��ô�ˮ������Һ��̼���ƺ����������������ᷴӦ��������������ˮ�к������ɵ��Ȼ��⣬���ʺ��������ᣬ��ѡABC��
��3���ٲⶨ��Һ������ʹ�õ�ʱpH��ֽ�����Ա����Ϊ��C��
��0.06gþԪ���൱�ڸ�Ԫ�ص�����Ϊ��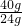 ��0.06g=0.1g������̼��Ƶ�����Ϊ��
��0.06g=0.1g������̼��Ƶ�����Ϊ�� ��0.2g=0.5g����ˮ��Ӳ��Ϊ��
��0.2g=0.5g����ˮ��Ӳ��Ϊ��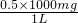 =500mg/L��450mg/�����������ñ������Ա����Ϊ�������ϣ�
=500mg/L��450mg/�����������ñ������Ա����Ϊ�������ϣ�
�������������е�֪ʶ���з���������̿���������ԣ�������ˮ�е�ɫ�غ���ζ�����ݻ��ϼ۹��������ö�����������Ԫ�صĻ��ϼۣ��ⶨ��Һ������ʹ�õ�ʱpH��ֽ����ŭ������ṩ�����ݼ��ɽ��м��㣮
���������⿼�龻ˮ���й�֪ʶ����ɴ��⣬�����������е�֪ʶ�������ṩ����Ϣ���У�
��2��������Ԫ�صĻ��ͼ�Ϊx��x+��-2����2=0�����x=+4�����Ա����Ϊ��+4��
�ڷ������в���������Ҳ������������ˮ���������Ա����Ϊ����������
������������ˮ��Ӧ���ɵ����ʷ�Ӧ�ģ����ʺ��ô�ˮ������Һ��̼���ƺ����������������ᷴӦ��������������ˮ�к������ɵ��Ȼ��⣬���ʺ��������ᣬ��ѡABC��
��3���ٲⶨ��Һ������ʹ�õ�ʱpH��ֽ�����Ա����Ϊ��C��
��0.06gþԪ���൱�ڸ�Ԫ�ص�����Ϊ��
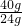 ��0.06g=0.1g������̼��Ƶ�����Ϊ��
��0.06g=0.1g������̼��Ƶ�����Ϊ�� ��0.2g=0.5g����ˮ��Ӳ��Ϊ��
��0.2g=0.5g����ˮ��Ӳ��Ϊ��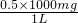 =500mg/L��450mg/�����������ñ������Ա����Ϊ�������ϣ�
=500mg/L��450mg/�����������ñ������Ա����Ϊ�������ϣ��������������е�֪ʶ���з���������̿���������ԣ�������ˮ�е�ɫ�غ���ζ�����ݻ��ϼ۹��������ö�����������Ԫ�صĻ��ϼۣ��ⶨ��Һ������ʹ�õ�ʱpH��ֽ����ŭ������ṩ�����ݼ��ɽ��м��㣮
���������⿼�龻ˮ���й�֪ʶ����ɴ��⣬�����������е�֪ʶ�������ṩ����Ϣ���У�

��ϰ��ϵ�д�
�����Ŀ
2006��ף��ҹ��䲼�µ���������ˮ���������ñ��а����������ݣ�
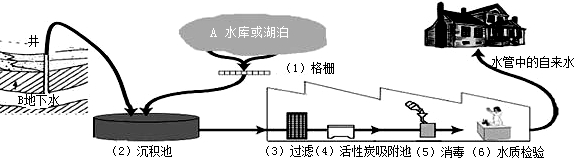
����סլ����ˮ����ͼ��ʾ���̴������ʼ����ﵽ��������ˮ����

��1��������ˮ�����У���������Ȼˮ����ζ���� ��
��2���������ȣ�ClO2����һ����ˮ�����ȷ����й㷺Ӧ�õĸ�Ч��ȫ����������������Cl2����ȣ��������Ȳ������и�ǿ��ɱ�����������Ҳ��������������DZ��Σ�����л��Ȼ��
�ٶ������ȣ�ClO2���У���Ԫ�صĻ��ϼ�Ϊ ��
���ڶ������ȵ��Ʊ������У�����������;����
����һ��2NaClO3+H2O2+H2SO4=2ClO2��+Na2SO4+O2��+2H2O
��������2NaClO3+4HCl=2ClO2��+Cl2��+2NaCl+2H2O
���������ַ����Ʊ��Ķ������ȣ����ʺ���������ˮ�������� �������һ��������������
����������ˮ������ʱ���������·�Ӧ��Cl2+H2O=HClO+HCl���������������������ˮ���ܶ���Ϊ1g/cm3������������������Ϊ1%��������Һ�����ʵ�����������Ӱ����� ������ĸ����
A��ϡ���� B��̼������Һ C������������Һ D���������Һ
��3��ˮ�ʼ���Ա�Ը���ָ����м���
�ټ��ˮ������Ӧ���� ������ţ���
A����ɫʯ����Һ B����ɫ��̪��Һ C��pH��ֽ
����Ȼˮ��һ�㶼����Ca2+��Mg2+�����ӣ���Ca2+��Mg2+�ܺ�����������ˮӲ�ȱ�ʱ����Ϊ���ϸ�ˮ�ʼ���Ա�ⶨ1Lij��������ˮ�к���Ԫ��0.1g��þԪ��0.06g������24gþԪ���൱��40g��Ԫ�ػ����Ӳ�ȣ��жϸ�ˮ�� ������ϡ������ϡ����ҹ�����ˮ���е���Ӳ�ȣ�
| ָ�� | �����Ŀ����λ | ��ֵ |
| ����ָ�� | �ܴ˾�Ⱥ��MPN/100ml�� | ���ü�� |
| �й���״ָ�� | ����ζ | �����ᡢ����ζ |
| ���ۿɼ��� | �� | |
| һ�㻯ѧָ�� | pH | ��С��6.5�Ҳ�����8.5 |
| ��Ӳ�ȣ���CaCO3�ƣ���mg/l�� | 450 | |
| �ܽ����ܹ��壨 mg/l�� | 1000 |

��1��������ˮ�����У���������Ȼˮ����ζ����
��2���������ȣ�ClO2����һ����ˮ�����ȷ����й㷺Ӧ�õĸ�Ч��ȫ����������������Cl2����ȣ��������Ȳ������и�ǿ��ɱ�����������Ҳ��������������DZ��Σ�����л��Ȼ��
�ٶ������ȣ�ClO2���У���Ԫ�صĻ��ϼ�Ϊ
���ڶ������ȵ��Ʊ������У�����������;����
����һ��2NaClO3+H2O2+H2SO4=2ClO2��+Na2SO4+O2��+2H2O
��������2NaClO3+4HCl=2ClO2��+Cl2��+2NaCl+2H2O
���������ַ����Ʊ��Ķ������ȣ����ʺ���������ˮ��������
����������ˮ������ʱ���������·�Ӧ��Cl2+H2O=HClO+HCl���������������������ˮ���ܶ���Ϊ1g/cm3������������������Ϊ1%��������Һ�����ʵ�����������Ӱ�����
A��ϡ���� B��̼������Һ C������������Һ D���������Һ
��3��ˮ�ʼ���Ա�Ը���ָ����м���
�ټ��ˮ������Ӧ����
A����ɫʯ����Һ B����ɫ��̪��Һ C��pH��ֽ
����Ȼˮ��һ�㶼����Ca2+��Mg2+�����ӣ���Ca2+��Mg2+�ܺ�����������ˮӲ�ȱ�ʱ����Ϊ���ϸ�ˮ�ʼ���Ա�ⶨ1Lij��������ˮ�к���Ԫ��0.1g��þԪ��0.06g������24gþԪ���൱��40g��Ԫ�ػ����Ӳ�ȣ��жϸ�ˮ��
��2010?˳������ģ��2006��ף��ҹ��䲼�µ���������ˮ���������ñ��а����������ݣ�
����סլ����ˮ����ͼ��ʾ���̴������ʼ����ﵽ��������ˮ����

��1��������ˮ�����У���������Ȼˮ����ζ���ǣ�
��2���������ȣ�ClO2����һ����ˮ�����ȷ����й㷺Ӧ�õĸ�Ч��ȫ����������������Cl2����ȣ��������Ȳ������и�ǿ��ɱ�����������Ҳ��������������DZ��Σ�����л��Ȼ��
�ٶ������ȣ�ClO2���У���Ԫ�صĻ��ϼ�Ϊ��
���ڶ������ȵ��Ʊ������У�����������;����
����һ��2NaClO3+H2O2+H2SO4=2ClO2��+Na2SO4+O2��+2H2O
��������2NaClO3+4HCl=2ClO2��+Cl2��+2NaCl+2H2O
���������ַ����Ʊ��Ķ������ȣ����ʺ���������ˮ�������ǣ������һ��������������
����������ˮ������ʱ���������·�Ӧ��Cl2+H2O=HClO+HCl���������������������ˮ���ܶ���Ϊ1g/cm3������������������Ϊ1%��������Һ�����ʵ�����������Ӱ����ǣ�����ĸ����
A��ϡ���� B��̼������Һ C������������Һ D���������Һ
��3��ˮ�ʼ���Ա�Ը���ָ����м���
�ټ��ˮ������Ӧ���ã�����ţ���
A����ɫʯ����Һ B����ɫ��̪��Һ C��pH��ֽ
����Ȼˮ��һ�㶼����Ca2+��Mg2+�����ӣ���Ca2+��Mg2+�ܺ�����������ˮӲ�ȱ�ʱ����Ϊ���ϸ�ˮ�ʼ���Ա�ⶨ1Lij��������ˮ�к���Ԫ��0.1g��þԪ��0.06g������24gþԪ���൱��40g��Ԫ�ػ����Ӳ�ȣ��жϸ�ˮ�� ������ϡ������ϡ����ҹ�����ˮ���е���Ӳ�ȣ�
| ָ�� | �����Ŀ����λ | ��ֵ |
| ����ָ�� | �ܴ˾�Ⱥ��MPN/100ml�� | ���ü�� |
| �й���״ָ�� | ����ζ | �����ᡢ����ζ |
| ���ۿɼ��� | �� | |
| һ�㻯ѧָ�� | pH | ��С��6.5�Ҳ�����8.5 |
| ��Ӳ�ȣ���CaCO3�ƣ���mg/l�� | 450 | |
| �ܽ����ܹ��壨 mg/l�� | 1000 |

��1��������ˮ�����У���������Ȼˮ����ζ���ǣ�
��2���������ȣ�ClO2����һ����ˮ�����ȷ����й㷺Ӧ�õĸ�Ч��ȫ����������������Cl2����ȣ��������Ȳ������и�ǿ��ɱ�����������Ҳ��������������DZ��Σ�����л��Ȼ��
�ٶ������ȣ�ClO2���У���Ԫ�صĻ��ϼ�Ϊ��
���ڶ������ȵ��Ʊ������У�����������;����
����һ��2NaClO3+H2O2+H2SO4=2ClO2��+Na2SO4+O2��+2H2O
��������2NaClO3+4HCl=2ClO2��+Cl2��+2NaCl+2H2O
���������ַ����Ʊ��Ķ������ȣ����ʺ���������ˮ�������ǣ������һ��������������
����������ˮ������ʱ���������·�Ӧ��Cl2+H2O=HClO+HCl���������������������ˮ���ܶ���Ϊ1g/cm3������������������Ϊ1%��������Һ�����ʵ�����������Ӱ����ǣ�����ĸ����
A��ϡ���� B��̼������Һ C������������Һ D���������Һ
��3��ˮ�ʼ���Ա�Ը���ָ����м���
�ټ��ˮ������Ӧ���ã�����ţ���
A����ɫʯ����Һ B����ɫ��̪��Һ C��pH��ֽ
����Ȼˮ��һ�㶼����Ca2+��Mg2+�����ӣ���Ca2+��Mg2+�ܺ�����������ˮӲ�ȱ�ʱ����Ϊ���ϸ�ˮ�ʼ���Ա�ⶨ1Lij��������ˮ�к���Ԫ��0.1g��þԪ��0.06g������24gþԪ���൱��40g��Ԫ�ػ����Ӳ�ȣ��жϸ�ˮ�� ������ϡ������ϡ����ҹ�����ˮ���е���Ӳ�ȣ�