��Ŀ����
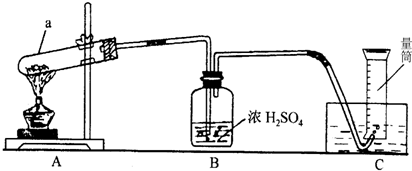
14����һ������MnO2��KMnO4��Ʒ��һλͬѧ�������ʵ��װ��ȥ�����䴿�ȣ�ȡ4.00g��Ʒ����ͼ�г�ּ��ȣ�ֱ����Ӧ��ȫ��������ͼ��װ�ò���������������������������ܶ�Ϊ1.41g/L������ˮ������������Բ��ƣ���ŨH2SO4������ˮ�ԣ��ڴ����������
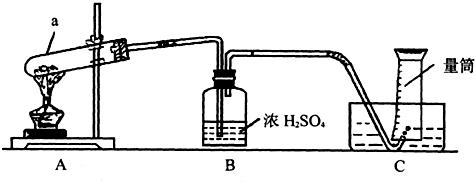
��ش𣺣�1��д��a�������ƣ�
��2��ָ��Aװ���е�һ�������ǣ�
��3����ȥBװ�ö�ʵ������û��Ӱ��
��4������A�Ĵ����A��װ�û�������������һ��������ȡ��
A�������Ȼ�粒������ʯ�ҵĻ������NH3
B������Ũ����Ͷ������̵Ļ������Cl2
C������ŨH2SO4�;ƾ��������C2H4����ϩ��
��5����Ӧ��������Ͳ��Һ�洦��227mL�̶ȴ�����Ͳ����Һ��߶�һ�£�������������Ϊ

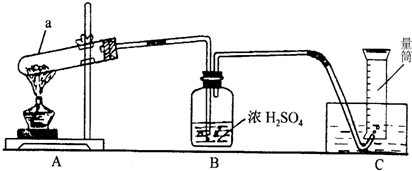
��ش𣺣�1��д��a�������ƣ�
�Թ�
����2��ָ��Aװ���е�һ�������ǣ�
�Թܿ�����
���ô��������ɵĺ��������ˮ�����������Թܵĵײ�ʹ�Թ�ը��
����3����ȥBװ�ö�ʵ������û��Ӱ��
��
����С����ޡ�����ԭ����װ��Ϊ��ˮ���ռ��������ʲ���Ҫ����
����4������A�Ĵ����A��װ�û�������������һ��������ȡ��
A
��A�������Ȼ�粒������ʯ�ҵĻ������NH3
B������Ũ����Ͷ������̵Ļ������Cl2
C������ŨH2SO4�;ƾ��������C2H4����ϩ��
��5����Ӧ��������Ͳ��Һ�洦��227mL�̶ȴ�����Ͳ����Һ��߶�һ�£�������������Ϊ
0.32g
g����������1����Ϥ�����������˽����ƣ�
��2�����ݸ��������ȡ������ע�����������
��3��������ˮ���ռ����������
��4������Aװ�����ڵķ�Ӧ���״̬������
��5�����ݹ�ʽm=��?v���㼴�ɣ�
��2�����ݸ��������ȡ������ע�����������
��3��������ˮ���ռ����������
��4������Aװ�����ڵķ�Ӧ���״̬������
��5�����ݹ�ʽm=��?v���㼴�ɣ�
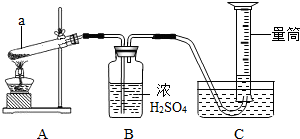
����⣺��1��ͼ��a�Ƿ�Ӧ�����Թܣ��ʴ�Ϊ���Թܣ�
��2��ʵ�����ø��������ȡ����ʱ��Ϊ��ֹ����Һ�������Թܣ�ʹ�Թ�ը�ѣ��Թܿ�Ӧ������б��
�ʴ�Ϊ���Թܿڳ��ϣ�������ˮ�����������Թܵĵײ�ʹ�Թ�ը�ѣ�
��3��Bװ����Ũ���������������ˮ�֣�����װ������ˮ���ռ�������������
�ʴ�Ϊ���ޣ���װ��Ϊ��ˮ���ռ��������ʲ���Ҫ���
��4��Aװ�����ڡ��̹̼��ȷ�Ӧ��������ֻ��A�ʺϣ�
��ѡA��
��5�����ݹ�ʽm=��?v��֪����������=l.41g/L?0.227L=0.32g��
�ʴ�Ϊ��0.32g��
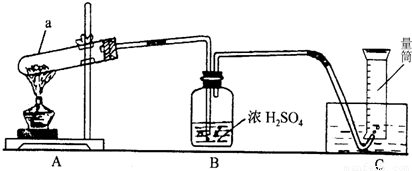
��2��ʵ�����ø��������ȡ����ʱ��Ϊ��ֹ����Һ�������Թܣ�ʹ�Թ�ը�ѣ��Թܿ�Ӧ������б��
�ʴ�Ϊ���Թܿڳ��ϣ�������ˮ�����������Թܵĵײ�ʹ�Թ�ը�ѣ�
��3��Bװ����Ũ���������������ˮ�֣�����װ������ˮ���ռ�������������
�ʴ�Ϊ���ޣ���װ��Ϊ��ˮ���ռ��������ʲ���Ҫ���
��4��Aװ�����ڡ��̹̼��ȷ�Ӧ��������ֻ��A�ʺϣ�
��ѡA��
��5�����ݹ�ʽm=��?v��֪����������=l.41g/L?0.227L=0.32g��
�ʴ�Ϊ��0.32g��
���������⿼��ʵ������ȡ�����ķ�����ԭ���������Լ���صļ��㣬��һ���ۺ��Ժ�ǿ����Ŀ���ۺϿ���ѧ���ķ������⡢��������������

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ