��Ŀ����
7������ѧ֪ʶ�ش��������⣮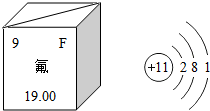 ��1����������ijЩ��������Ӽ�������ЧԤ��ȣ�ݣ���ͼ�Ƿ�Ԫ�������ڱ��е������Ϣ����ԭ�ӽṹʾ��ͼ�����Ԫ�ص�ԭ�Ӻ����������9����ԭ���ڻ�ѧ��Ӧ����ʧ���ʧ���á������ӣ������ƿ��������������F2����Ӧ�Ƶã���Ӧ�Ļ�ѧ����ʽ��2Na+F2=2NaF��
��1����������ijЩ��������Ӽ�������ЧԤ��ȣ�ݣ���ͼ�Ƿ�Ԫ�������ڱ��е������Ϣ����ԭ�ӽṹʾ��ͼ�����Ԫ�ص�ԭ�Ӻ����������9����ԭ���ڻ�ѧ��Ӧ����ʧ���ʧ���á������ӣ������ƿ��������������F2����Ӧ�Ƶã���Ӧ�Ļ�ѧ����ʽ��2Na+F2=2NaF����2���⡢̼�������Ƶ�Ԫ������ɶ������ʣ�A�Ļ�ѧʽ��CaO���׳���ʯ�ң�A��B��θҺ�к��е��ᣩ��Ӧ�ų��������ȣ��÷�Ӧ�Ļ�ѧ����ʽ��CaO+2HCl=CaCl2+H2O���û�ѧʽ��ʾ3��̼�������3CO32-��
���� ��1������Ԫ�����ڱ���ԭ�ӽṹʾ��ͼ����Ϣ��������𣻣�2���������ʵ��׳ơ���ѧ����ʽ����д�����Լ����ӵı�ʾ�������������
��� �⣺��1����ԭ���У�ԭ������=�˵����=������=�������������Ԫ�ص�ԭ������Ϊ9������ԭ�Ӻ�����9�����ӣ�����ԭ�ӽṹʾ��ͼ��֪�����������1�����ӣ��ڻ�ѧ�仯����ʧȥ������1�����ӣ�ͨ����+1�ۣ���ԭ���������7�����ӣ��õ�1�����Ӷ���-1�ۣ��ʶ�����ɵĻ�����Ļ�ѧʽΪNaF�����9��ʧ��2Na+F2=2NaF��
��2��CaO�������ƵĻ�ѧʽ���׳���ʯ�ң�θ��ijɷ������ᣬ���߷�Ӧ�����Ȼ��ƺ�ˮ���������ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣����3��̼������ӱ�ʾΪ��3CO32-��
�����ʯ�ң�CaO+2HCl=CaCl2+H2O��3CO32-��
���� �����ѶȲ�����ѧ���������Ԫ�����ڱ���Ԫ�ص���Ϣ��ԭ�ӽṹʾ��ͼ�Ƚ��з��������������

��ϰ��ϵ�д�
 ����������ϵ�д�
����������ϵ�д�
�����Ŀ
17�����ж�һЩ��ʵ�Ľ��Ͳ���ȷ���ǣ�������
| �� ʵ | �� �� | |
| A | ��̼��īˮ��д���� | �����µ���̼�Ļ�ѧ�����ȶ� |
| B | ʯī�ܹ�����صĵ缫���� | ʯī���� |
| C | ��̿�������������������ʯ�ﻹԭ���� | ��̿���л�ԭ�� |
| D | ���ǹ�ҵ���û���̿����ɫ���ư��� | ����̿���������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
18����ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ�������й�ʵ�������˵������ȷ���ǣ�������
| A�� | ���������������������ʵ�ȥ����ʵ��ʱ��һ��Ҫ�õ����˲��� | |
| B�� | ̼���Ρ�̼��������ֻ��̼�����������ᷴӦ����CO2 | |
| C�� | ����������һ�������Ż�ѧ�仯 | |
| D�� | ��ʹ��ɫ��̪��Һ��������һ���Ǽ� |
15�������й���Һ��˵����ȷ���ǣ�������
| A�� | ���ƺ�6%��NaCl��Һ��װƿʱ��С����©һ���֣�ƿ��NaCl��ҺŨ����Ϊ6% | |
| B�� | ���ڷ��ú�ֲ��Һ��һ������Һ | |
| C�� | KNO3������Һһ���Ȳ�������Һ�������������� | |
| D�� | ���ͱ�����Һ���¶ȣ�һ���о������� |
2��Ϊ��̽��a��b��c���ֽ������˳��ijͬѧ���������ʵ�飮ʵ�������ȷ���ǣ�������


| A�� | b��a��c | B�� | a��b��c | C�� | c��b��a | D�� | c��a��b |
12�����г�������У��漰��ѧ�仯���ǣ�������
| A�� | ������� | B�� | �����ˮ | C�� | �����ɸ� | D�� | ��ľ�Ա� |
8������˵����ȷ���ǣ�������
| A�� | ������ˮ��ϴ���г����Է��� | B�� | ������Ҫ�ɵ������������ | ||
| C�� | �û���̿���Խ���ˮ��Ӳ�� | D�� | ���Ͻ���һ�ִ����� |
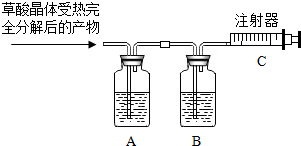 ��Щ���尴��������һ�����Ľᾧˮ�����нᾧˮ�ľ�����һ���¶��»���ȥ�ᾧˮ�����ᾧ�壨H2C2O4•xH2O���ڳ�������һ����ɫ�����壮���ᾧ������ʱ�ֽ�Ļ�ѧ����ʽΪ��
��Щ���尴��������һ�����Ľᾧˮ�����нᾧˮ�ľ�����һ���¶��»���ȥ�ᾧˮ�����ᾧ�壨H2C2O4•xH2O���ڳ�������һ����ɫ�����壮���ᾧ������ʱ�ֽ�Ļ�ѧ����ʽΪ��