��Ŀ����
����¹�̷��¼����ġ�Ԫ�ס��������谷���仯ѧʽΪC3H6N6�������谷������������ԭ�ϣ�����ˮ���������ԣ������谷��Ħ��������________������̼���⡢��ԭ�ӵĸ�����Ϊ________��̼����Ԫ��������Ϊ________��1molC3H6N6��Լ��̼ԭ�ӵĸ���Ϊ________���������谷�ĺ������ϸߣ����Ա�������������������Ʒ�У��ԡ���ߡ������ʺ����������谷�е�Ԫ����������Ϊ________������ȷ��0.1%��
126g/mol 1��2��2 6��1 1.806��1024 66.7%
����������1Ħ�κ����ʵ����������Կ�Ϊ��λ����ֵ�ϵ��ڸ���ԭ�ӵ����ԭ�����������н��
���ݱ���Ԫ�ط������½ǵ����ֱ�ʾһ������������ԭ�ӵĸ��������н��
���ݻ������и�Ԫ��������=��Ԫ�ص����ԭ��������ԭ�Ӹ���֮�ȣ����н��
����ÿ1Ħ���κ����ʺ��а���٤��������Լ6.02��1023�����������н��
����������ijԪ�ص���������= ��100%�����н��
��100%������
���
����1Ħ�κ����ʵ����������Կ�Ϊ��λ����ֵ�ϵ��ڸ���ԭ�ӵ����ԭ����������֪�����谷��Ħ������Ϊ��12��3+6+14��6=126g/mol�� �ʴ�Ϊ��126g/mol��
���ݱ���Ԫ�ط������½ǵ����ֱ�ʾһ������������ԭ�ӵĸ������ɵ�C3H6N6��̼���⡢��ԭ�ӵĸ�����Ϊ��3��6��6=1��2��2��
���ݻ������и�Ԫ��������=��Ԫ�ص����ԭ��������ԭ�Ӹ���֮�ȣ��ɵ�C3H6N6��̼����Ԫ�ص�������Ϊ����12��3����6=6��1��
����ÿ1Ħ���κ����ʺ��а���٤��������Լ6.02��1023���������ɵ�
1molC3H6N6��Լ��̼ԭ�ӵĸ���Ϊ��6.02��1023��3=1.806��1024��
����������ijԪ�ص���������= ��100%���ɵ�
��100%���ɵ�
�����谷�е�Ԫ����������= ��100%=66.7%��
��100%=66.7%��
�ʴ�Ϊ��126g/mol��
1��2��2��
6��1��
1.806��1024��
66.7%��
���������⿼��ѧ�������ʵ��������Ӹ���֮��Ĺ�ϵ�����������գ�����������Ԫ�ص���������ԭ�Ӹ����ȣ�
����������1Ħ�κ����ʵ����������Կ�Ϊ��λ����ֵ�ϵ��ڸ���ԭ�ӵ����ԭ�����������н��
���ݱ���Ԫ�ط������½ǵ����ֱ�ʾһ������������ԭ�ӵĸ��������н��
���ݻ������и�Ԫ��������=��Ԫ�ص����ԭ��������ԭ�Ӹ���֮�ȣ����н��
����ÿ1Ħ���κ����ʺ��а���٤��������Լ6.02��1023�����������н��
����������ijԪ�ص���������=
 ��100%������
��100%���������
����1Ħ�κ����ʵ����������Կ�Ϊ��λ����ֵ�ϵ��ڸ���ԭ�ӵ����ԭ����������֪�����谷��Ħ������Ϊ��12��3+6+14��6=126g/mol�� �ʴ�Ϊ��126g/mol��
���ݱ���Ԫ�ط������½ǵ����ֱ�ʾһ������������ԭ�ӵĸ������ɵ�C3H6N6��̼���⡢��ԭ�ӵĸ�����Ϊ��3��6��6=1��2��2��
���ݻ������и�Ԫ��������=��Ԫ�ص����ԭ��������ԭ�Ӹ���֮�ȣ��ɵ�C3H6N6��̼����Ԫ�ص�������Ϊ����12��3����6=6��1��
����ÿ1Ħ���κ����ʺ��а���٤��������Լ6.02��1023���������ɵ�
1molC3H6N6��Լ��̼ԭ�ӵĸ���Ϊ��6.02��1023��3=1.806��1024��
����������ijԪ�ص���������=
 ��100%���ɵ�
��100%���ɵ������谷�е�Ԫ����������=
 ��100%=66.7%��
��100%=66.7%�� �ʴ�Ϊ��126g/mol��
1��2��2��
6��1��
1.806��1024��
66.7%��
���������⿼��ѧ�������ʵ��������Ӹ���֮��Ĺ�ϵ�����������գ�����������Ԫ�ص���������ԭ�Ӹ����ȣ�

��ϰ��ϵ�д�
 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д�
�����Ŀ
 ��ȫ���ġ���¹�̷ۡ��¼��е���������������谷�����ӽṹ��ͼ��ʾ���������谷��һ����Ҫ�Ļ���ԭ�ϣ������京�����ߣ���Ŀǰ��������ͨ�á�������궨ţ���е����ʺ���������һЩ�������˾ͽ�������̷����ԡ���ߡ��̷۵�Ʒ�ʣ�
��ȫ���ġ���¹�̷ۡ��¼��е���������������谷�����ӽṹ��ͼ��ʾ���������谷��һ����Ҫ�Ļ���ԭ�ϣ������京�����ߣ���Ŀǰ��������ͨ�á�������궨ţ���е����ʺ���������һЩ�������˾ͽ�������̷����ԡ���ߡ��̷۵�Ʒ�ʣ�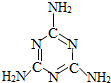 ��ȫ���ġ���¹�̷ۡ��¼��е���������������谷�����ӽṹ��ͼ���������谷��һ����Ҫ�Ļ���ԭ�ϣ������京�����ߣ���Ŀǰ��������ͨ�á�������궨ţ���е����ʺ���������һЩ�������˾ͽ�������̷����ԡ���ߡ��̷۵�Ʒ�ʣ�
��ȫ���ġ���¹�̷ۡ��¼��е���������������谷�����ӽṹ��ͼ���������谷��һ����Ҫ�Ļ���ԭ�ϣ������京�����ߣ���Ŀǰ��������ͨ�á�������궨ţ���е����ʺ���������һЩ�������˾ͽ�������̷����ԡ���ߡ��̷۵�Ʒ�ʣ� ��2009?������һģ����ȫ���ġ���¹�̷ۡ��¼��е���������������谷�����ӽṹ��ͼ���������谷��һ����Ҫ�Ļ���ԭ�ϣ������京�����ߣ���Ŀǰ��������ͨ�á�������궨ţ���е����ʺ���������һЩ�������˾ͽ�������̷����ԡ���ߡ��̷۵�Ʒ�ʣ�
��2009?������һģ����ȫ���ġ���¹�̷ۡ��¼��е���������������谷�����ӽṹ��ͼ���������谷��һ����Ҫ�Ļ���ԭ�ϣ������京�����ߣ���Ŀǰ��������ͨ�á�������궨ţ���е����ʺ���������һЩ�������˾ͽ�������̷����ԡ���ߡ��̷۵�Ʒ�ʣ� ��ȫ���ġ���¹�̷ۡ��¼��е���������������谷�����ӽṹ��ͼ��ʾ���������谷��һ����Ҫ�Ļ���ԭ�ϣ������京�����ߣ���Ŀǰ��������ͨ�á�������궨ţ���е����ʺ���������һЩ�������˾ͽ�������̷����ԡ���ߡ��̷۵�Ʒ�ʣ�
��ȫ���ġ���¹�̷ۡ��¼��е���������������谷�����ӽṹ��ͼ��ʾ���������谷��һ����Ҫ�Ļ���ԭ�ϣ������京�����ߣ���Ŀǰ��������ͨ�á�������궨ţ���е����ʺ���������һЩ�������˾ͽ�������̷����ԡ���ߡ��̷۵�Ʒ�ʣ�