��Ŀ����
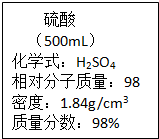 ʵ����һƿŨ������̱���ͼ��ʾ�������й���Ϣ���㣮
ʵ����һƿŨ������̱���ͼ��ʾ�������й���Ϣ���㣮��1������ǰ��ƿŨ������Һ��������
��2��ȡ������Һ50�ˣ�ϡ�ͳ�20%��ϡ���ᣬ���ˮ���ٿˣ�
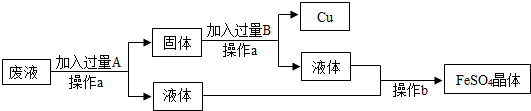
��3��ij�о���ѧϰС�����ⶨ������ķ�Һ�����������������������������ʵ�飺��100g�÷�Һ�м��������ķ���м����ַ�Ӧ���ռ�������0.2g�������Һ�������ɷֲ������м��Ӧ�����������÷�Һ�����������������������Ҫ��д��������̣�
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,��ˮϡ�ı�Ũ�ȵķ���,�й��������������ļ���
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
��������1���ɱ�ǩ�ɶ�ȡ��ƿŨ��������500mL���ܶ�1.84g/cm3������m=��V�ɼ����ƿŨ�����������
��2����98%��Ũ��������20%��ϡ������Ҫ��ˮ����ϡ�ͣ�����ϡ��ǰ����Һ�������������䣬��ϡ��ǰŨ�����������������Ƶ�ϡ�����������
��3�������������ᷴӦ�Ļ�ѧ����ʽ�������������������г�����ʽ���Ϳɼ�����÷�Һ���������ʵ�������Ȼ����ݡ�������������=
��100%�����㼴�ɣ�
��2����98%��Ũ��������20%��ϡ������Ҫ��ˮ����ϡ�ͣ�����ϡ��ǰ����Һ�������������䣬��ϡ��ǰŨ�����������������Ƶ�ϡ�����������
��3�������������ᷴӦ�Ļ�ѧ����ʽ�������������������г�����ʽ���Ϳɼ�����÷�Һ���������ʵ�������Ȼ����ݡ�������������=
| �������� |
| ��Һ���� |
����⣺��1���ɱ�ǩ��֪��ƿŨ��������500mL���ܶ�1.84g/cm3������ǰ��ƿŨ������Һ������=500mL��1.84g/cm3=920g
��2�����Ƴ�20%��ϡ�������ˮ������=
-50g=195g
��3����÷�Һ���������ʵ�����Ϊx��
Fe+H2SO4=FeSO4+H2��
98 2
x 0.2g
=
x=9.8g
��÷�Һ���������������Ϊ��
��100%=9.8%��
�𣺸÷�Һ���������������Ϊ9.8%��
�ʴ�Ϊ����1��920����2��195g����3��9.8%��
��2�����Ƴ�20%��ϡ�������ˮ������=
| 50g��98% |
| 20% |
��3����÷�Һ���������ʵ�����Ϊx��
Fe+H2SO4=FeSO4+H2��
98 2
x 0.2g
| 98 |
| x |
| 2 |
| 0.2g |
x=9.8g
��÷�Һ���������������Ϊ��
| 9.8g |
| 100g |
�𣺸÷�Һ���������������Ϊ9.8%��
�ʴ�Ϊ����1��920����2��195g����3��9.8%��
������������Һ����������=
��100%��������������������֪���ɼ��������Һ��صĵ���������
| �������� |
| ��Һ���� |

��ϰ��ϵ�д�
�����Ŀ
�������������ڴ�������ǣ�������
| A����Ȼ��Ȫˮ |
| B���糿���ʵĿ��� |
| C������������ˮ |
| D��������̼���� |
ij���嵥�ʵ���Է�����������������Է�������֮��Ϊ7��8���������ǣ�������
| A������ | B������ |
| C������ | D��һ����̼ |

 ͨ�����л�ѧ��ѧϰ������֪��ͨ������;���ɻ��������
ͨ�����л�ѧ��ѧϰ������֪��ͨ������;���ɻ��������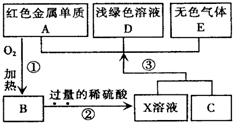 A��B��C��D��EΪ���л�ѧ���������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�
A��B��C��D��EΪ���л�ѧ���������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�