��Ŀ����
22��Ϊ��̽����ˮ�ľ��������̣�ijʵ��С��ӻ��Ǻ���ȡ��ˮ�����۲쵽��
A��ˮ���ʻ�ɫ������ζ B��ˮ�����ǣ��й���С�������ֶ�ˮ���������´�����
��1����ˮ���м������������ܽ⣬����һ��ʱ�����
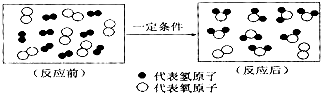
��2��ͨ������ɱ���������õ�����ˮ�������ķ�Ӧ���£�Cl2+H2O=HCl+HClO�������ᣩ�����жϣ�����������Ԫ�صĻ��ϼ�Ϊ
��3��Ϊ���жϵõ�������ˮ��Ӳˮ����ˮ���ɼ���
A��ˮ���ʻ�ɫ������ζ B��ˮ�����ǣ��й���С�������ֶ�ˮ���������´�����
��1����ˮ���м������������ܽ⣬����һ��ʱ�����
����
����������ƣ�����ȥ����С������������Һ�м������̿������������
�Գ�ȥˮ���е���ɫ����ζ���ù�����������
�仯�����������ѧ��������2��ͨ������ɱ���������õ�����ˮ�������ķ�Ӧ���£�Cl2+H2O=HCl+HClO�������ᣩ�����жϣ�����������Ԫ�صĻ��ϼ�Ϊ
+1
����3��Ϊ���жϵõ�������ˮ��Ӳˮ����ˮ���ɼ���
����ˮ
���м��飮�����п�ͨ���������
��ת��Ӳˮ����������1�����˿��Ѳ�����ˮ�����ʳ�ȥ������̿�����������ã��ܹ�����ˮ�е�ɫ�غ���ζ��
��2���������У�Ԫ�ػ��ϼ۵Ĵ�����Ϊ�㣻
��3���÷���ˮ���Լ���ˮ����Ӳ��������п�������ˮ��
��2���������У�Ԫ�ػ��ϼ۵Ĵ�����Ϊ�㣻
��3���÷���ˮ���Լ���ˮ����Ӳ��������п�������ˮ��
����⣺��1�����˿��Գ�ȥˮ�еĹ���С���������û���̿�������Կ��Գ�ȥˮ���е���ɫ����ζ������̿������������û�����������ɣ����������仯��������ˣ�������������
��2���ڴ������У���Ԫ�صĻ��ϼ���+1����Ԫ�صĻ��ϼ���-2�����������Ԫ�صĻ��ϼ���+1�����+1��
��3����ˮ�м������ˮʱ����������϶����ĭ��˵������ˮ�������������ĭ���ٻ�����ĭ��˵����Ӳˮ���������ˮ��
�������ʱ��ˮ�еĸ����ӡ�þ�����ܹ���̼��ơ�������þ��������ʽ��ˮ�����������������У�
��2���ڴ������У���Ԫ�صĻ��ϼ���+1����Ԫ�صĻ��ϼ���-2�����������Ԫ�صĻ��ϼ���+1�����+1��
��3����ˮ�м������ˮʱ����������϶����ĭ��˵������ˮ�������������ĭ���ٻ�����ĭ��˵����Ӳˮ���������ˮ��
�������ʱ��ˮ�еĸ����ӡ�þ�����ܹ���̼��ơ�������þ��������ʽ��ˮ�����������������У�
������������Ҫ���龻��ˮ�ķ����ͻ��ϼ۵ȷ����֪ʶ�����ʱҪ�����ڵ����У�Ԫ�ػ��ϼ�Ϊ�㣬�ڻ������У�����Ԫ�صĻ��ϼ۲�Ϊ�㣬���Ǵ�����Ϊ�㣮

��ϰ��ϵ�д�
�����Ŀ
 ����ʱ��1��13��05��53���ط�����7.0��ǿ���Ѹ���������������ѣ�һ�������Ԯ��Աֱ�ԣ������ڽ�Ǯ���������ˮ���ǻ��ҡ��������ǹ���ˮ���о�����ش��й����⣺
����ʱ��1��13��05��53���ط�����7.0��ǿ���Ѹ���������������ѣ�һ�������Ԯ��Աֱ�ԣ������ڽ�Ǯ���������ˮ���ǻ��ҡ��������ǹ���ˮ���о�����ش��й����⣺ ij��ѧ��ȤС��Ϊ��̽����ˮ�ľ��������̣��ӻ��Ǻ���ȡ��ˮ�����۲쵽��ˮ���ʻ�ɫ������ζ�����ǣ��й���С�������ֶ�ˮ���������´�����
ij��ѧ��ȤС��Ϊ��̽����ˮ�ľ��������̣��ӻ��Ǻ���ȡ��ˮ�����۲쵽��ˮ���ʻ�ɫ������ζ�����ǣ��й���С�������ֶ�ˮ���������´�����