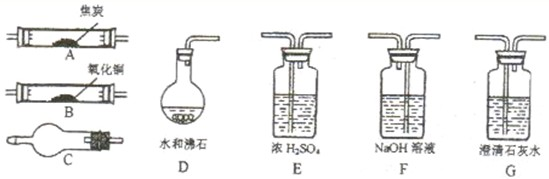
��Ŀ����
��ҵ��ӿˮ����ͨ�����ȵĽ�̿��Ӧ����������ɷ���Ҫ����CO��H2������CO2��H2O����������ͼ�ṩ��������ѡ���Ҫ���Լ������һ��ʵ��֤���������������һ������CO��H2������װ�ú͵��ܵ���;�о���ȥ��װ��C���ܼ��ȣ�

�ش��������⣺
��1����ȥ��������е�ˮ����ѡ��װ��______��ʢNaOH��Һװ����;��______��
��2��װ��B���������ķ�Ӧ����ʽ��______��
��3������ˮ����������װ�ø����C�м�����Լ��ǣ�д��ѧʽ��______��
��4�������������������A��G��ĸ���Ӹ�������˳��Ϊ��ÿ��װ��ֻ��һ�Σ�______
��5����֤����������к�CO��ʵ��������______��
��6����֤����������к�H2��ʵ��������______��
ʵ��˼��������ʵ��װ��ʱ���ܷ�װ��E��F˳���������ܡ����ܡ���______��

�ش��������⣺
��1����ȥ��������е�ˮ����ѡ��װ��______��ʢNaOH��Һװ����;��______��
��2��װ��B���������ķ�Ӧ����ʽ��______��
��3������ˮ����������װ�ø����C�м�����Լ��ǣ�д��ѧʽ��______��
��4�������������������A��G��ĸ���Ӹ�������˳��Ϊ��ÿ��װ��ֻ��һ�Σ�______
��5����֤����������к�CO��ʵ��������______��
��6����֤����������к�H2��ʵ��������______��
ʵ��˼��������ʵ��װ��ʱ���ܷ�װ��E��F˳���������ܡ����ܡ���______��
��1��Ũ���������ˮ�ԣ����Գ�ȥ��������е�ˮ����������������Һ�������������еĶ�����̼������ѧ��Ӧ���Ӷ���ȥ���еĶ�����̼���壻
���E����ȥCO2��
��2��װ��B���������ķ�Ӧ��������ԭ����ͭ���仯ѧ��Ӧ�ķ���ʽΪ��CuO+H2
Cu+H2O��
���CuO+H2
Cu+H2O��
��3����ɫ����ˮ����ͭ��ˮ���Ϊ��ɫ�����Կ���װ�ø����C�м�����ˮ����ͭ��CuSO4����ͨ���۲���ɫ�Ƿ�ı���ȷ��ˮ�����Ĵ��ڣ�
���CuSO4��
��4��Ϊ����֤������к���CO��H2��ʵ�������������Ⱥ�˳��Ϊ����������̼������ˮ�֡���ԭװ�á�����ˮ�֡����������̼��
���D��A��F��E��B��C��G��
��5��֤��ԭ���������CO���ڵ�ʵ��������D�к�ɫ��ĩ��죬G�г���ʯ��ˮ����ǣ���
���ԭ���������CO2�ѳ�ȥ������CO��CuO��Ӧ���ɵ�CO2ʹ�����ʯ��ˮ����ǣ�
��6��������������ڣ������ͺ�ɫ������ͭ��Ӧ���ɺ�ɫ��ͭ��ˮ��ˮ�Ͱ�ɫ����ˮ����ͭ��Ӧ������ɫ������ͭ���壻����װ�û�λ�ã�����������ͨ������������Һ������ˮ�ֳ�����Ӱ��Bװ���е�ʵ�飻
���ԭ���������H2�ѱ���ȥ������H2��CuO��Ӧ���ɵ�H2Oʹ��ˮ����ͭ�ɰ�ɫ��Ϊ��ɫ�����ܣ�
���E����ȥCO2��
��2��װ��B���������ķ�Ӧ��������ԭ����ͭ���仯ѧ��Ӧ�ķ���ʽΪ��CuO+H2
| ||
���CuO+H2
| ||
��3����ɫ����ˮ����ͭ��ˮ���Ϊ��ɫ�����Կ���װ�ø����C�м�����ˮ����ͭ��CuSO4����ͨ���۲���ɫ�Ƿ�ı���ȷ��ˮ�����Ĵ��ڣ�
���CuSO4��
��4��Ϊ����֤������к���CO��H2��ʵ�������������Ⱥ�˳��Ϊ����������̼������ˮ�֡���ԭװ�á�����ˮ�֡����������̼��
���D��A��F��E��B��C��G��
��5��֤��ԭ���������CO���ڵ�ʵ��������D�к�ɫ��ĩ��죬G�г���ʯ��ˮ����ǣ���
���ԭ���������CO2�ѳ�ȥ������CO��CuO��Ӧ���ɵ�CO2ʹ�����ʯ��ˮ����ǣ�
��6��������������ڣ������ͺ�ɫ������ͭ��Ӧ���ɺ�ɫ��ͭ��ˮ��ˮ�Ͱ�ɫ����ˮ����ͭ��Ӧ������ɫ������ͭ���壻����װ�û�λ�ã�����������ͨ������������Һ������ˮ�ֳ�����Ӱ��Bװ���е�ʵ�飻
���ԭ���������H2�ѱ���ȥ������H2��CuO��Ӧ���ɵ�H2Oʹ��ˮ����ͭ�ɰ�ɫ��Ϊ��ɫ�����ܣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ