��Ŀ����
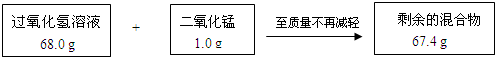
��8�֣�ij��ȤС��Ϊ�ⶨʵ������һƿ���õĹ���������Һ�����ʵ�����������ʵ���������������ͼ��ʾ��

��1��ʣ��Ļ�����ж������̵�����Ϊ g����Ӧ��������������Ϊ g��
��2������ù���������Һ����������������
��3��ʵ���������ͭ��Һ�ڸ÷�Ӧ��Ҳ���������̵����á�����5.6g��ˮ����ͭ����������̽��б�ʵ�飬����ʣ�����Һ�����ʵ�����������
������ͭȫ������ˮ������ʱ������0.1%��
(1)1.0�� 1.6�� �������������������� ��ÿ��1�֣���2�֣�
��2����68.0 g����������Һ�к��й������������Ϊx��
2H2O22H2O+O2�� ��1�֣�
68 32
x 1.6g
68��32 = x �� 1.6g ��1�֣�
x = 3.4g ��1�֣�
����������Һ�����ʵ���������= 3.4g/68.0g ��100% =5.0% (1��)
��3������ͭ��Һ��������������= 5.6g / (67.4g�� 1.0g + 5.6g) ��100% ��1�֣�
��7.8% ��1�֣�
����:��

 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�