��Ŀ����
��ѧ��һ����ʵ��Ϊ������ѧ�ƣ���ѧ��ȡ�õķ�˶�ɹ�������ʵ�����Ҫ���÷ֲ����ģ��������ʵ��װ��ͼ�ش����⣺

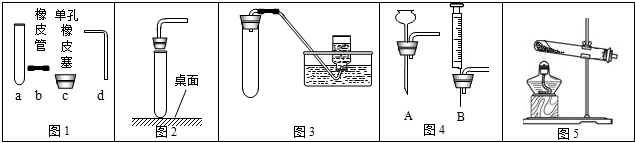
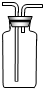
��1��д��ͼ�д�������������ƣ���______����______��
��2����ʵ���Ҷ��㿪�ţ����ͼ1��ѡ����������ȡ�����װ�ã���ȡһ������Ϥ�����壮������ȡ��������______����Ӧ�Ļ�ѧ����ʽΪ______����������巽����______��ѡ����װ�õ�������______������ѡ�����______������ţ���ͬ����ѡ����ռ�װ����______��ѡ����ռ�װ�õ�������______��
��3��װ��B��Ȼ������㣬�������Ʒ�Ӧ�ķ���ֹͣ�����ͼ2��ѡȡ______������ţ�ȡ��B�еĵ��������Դﵽ���Ʒ�Ӧ��Ŀ�ģ�
��4����D�ռ�����ʱ�������ܿ�����______ ʱ��ʼ�ռ�����______ ʱ���������ռ����ˣ�
��5��ʵ������20g�ĸ��������ȡ��������Ӧһ��ʱ����Թ��л�ʣ19.36g���壬���������������ٿˣ��ж��ٿ˸�����زμ��˷�Ӧ��
�⣺��1��ֱ��д������������Ϊ���پƾ��ƣ��ڳ���©����
��2������ȡ����ѡ����װ��ʱ��ͨ�����ݷ�Ӧ���״̬�ͷ�Ӧ������ѡ����װ�ã���װ��AΪ�̹̼����͵ķ���װ�ã�������������ѧ֪ʶ����֪����ʵ�������ø�����ػ���������������ʺϸ�װ�ã�����������ȡ���������������ܹ�֧��ȼ�յ����ʣ������ô����ǵ�ľ������������壻�������ܶȱȿ��������Կ���ѡ�������ſ��������ռ�����������������ˮ�����Կ���ѡ����ˮ�����ռ���
��3������ͼ2��װ�õ��ص������շ�������ԭ������֪��װ��b�����ʺϿ��Ʒ�Ӧ��ֹͣ�ͷ�����
��4������ˮ���ռ�����ʱӦ�õ����������ȵ�����ð��ʱ�����ռ���
��5����������������غ㶨�ɿ�����������ɵ�������������20g-19.36g=0.64g��
��μӷ�Ӧ�ĸ����������Ϊx
2KMnO4 K2MnO4+MnO2+O2��
K2MnO4+MnO2+O2��
316 32
x 0.64g

��ã�x=6.32g
������������������0.64g
�ʴ�Ϊ����1���پƾ��ƣ��ڳ���©����
��2��������2KMnO4 K2MnO4+MnO2+O2����2KClO3
K2MnO4+MnO2+O2����2KClO3 2KCl+3O2���� �ô����ǵ�ľ�����ڼ���ƿ�ڣ�ľ����ȼ����������Ӧ�����ͷ�Ӧ���״̬��A��C�� D�������ܶȱȿ������Ҳ�������ˮ��
2KCl+3O2���� �ô����ǵ�ľ�����ڼ���ƿ�ڣ�ľ����ȼ����������Ӧ�����ͷ�Ӧ���״̬��A��C�� D�������ܶȱȿ������Ҳ�������ˮ��
��3��a��
��4�����������ȷų�������ƿ���������ݳ���
��5���μӷ�Ӧ�ĸ������������6.32g��
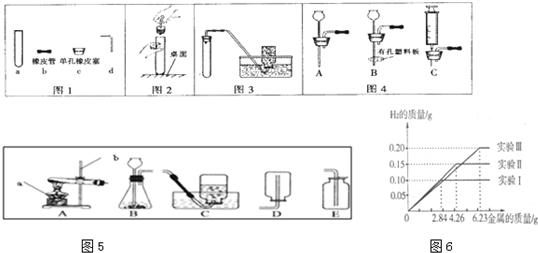
��������1��ֱ��д�����������ƣ�
��2��װ��AΪ�̹̼����͵ķ���װ�ã����Կ���������ȡ���������������ܹ�֧��ȼ�յ����ʣ������ô����ǵ�ľ������������壻���ݷ�Ӧ�����ͷ�Ӧ���״̬��ѡ����װ�ã�ѡ��������ռ�����ͨ���������ܶȺ�ˮ���ԣ�
��3���������շ��������ŵ���ѡ����ʵ�װ�ã�
��4������ˮ���ռ�����ʱӦ�õ����������ȵ�����ð��ʱ�����ռ���
��5�����������غ㶨�ɿ�����������ɵ�������������Ȼ����ݷ�Ӧ�Ļ�ѧ����ʽ��������������������������ص�������
���������⿼������������ȡԭ����װ�õ�ѡ��ֻҪ�����������ȡԭ��������װ��ѡ������ݣ��������ӭ�ж��⣮
��2������ȡ����ѡ����װ��ʱ��ͨ�����ݷ�Ӧ���״̬�ͷ�Ӧ������ѡ����װ�ã���װ��AΪ�̹̼����͵ķ���װ�ã�������������ѧ֪ʶ����֪����ʵ�������ø�����ػ���������������ʺϸ�װ�ã�����������ȡ���������������ܹ�֧��ȼ�յ����ʣ������ô����ǵ�ľ������������壻�������ܶȱȿ��������Կ���ѡ�������ſ��������ռ�����������������ˮ�����Կ���ѡ����ˮ�����ռ���
��3������ͼ2��װ�õ��ص������շ�������ԭ������֪��װ��b�����ʺϿ��Ʒ�Ӧ��ֹͣ�ͷ�����
��4������ˮ���ռ�����ʱӦ�õ����������ȵ�����ð��ʱ�����ռ���
��5����������������غ㶨�ɿ�����������ɵ�������������20g-19.36g=0.64g��
��μӷ�Ӧ�ĸ����������Ϊx
2KMnO4
 K2MnO4+MnO2+O2��
K2MnO4+MnO2+O2�� 316 32
x 0.64g

��ã�x=6.32g
������������������0.64g
�ʴ�Ϊ����1���پƾ��ƣ��ڳ���©����
��2��������2KMnO4
 K2MnO4+MnO2+O2����2KClO3
K2MnO4+MnO2+O2����2KClO3 2KCl+3O2���� �ô����ǵ�ľ�����ڼ���ƿ�ڣ�ľ����ȼ����������Ӧ�����ͷ�Ӧ���״̬��A��C�� D�������ܶȱȿ������Ҳ�������ˮ��
2KCl+3O2���� �ô����ǵ�ľ�����ڼ���ƿ�ڣ�ľ����ȼ����������Ӧ�����ͷ�Ӧ���״̬��A��C�� D�������ܶȱȿ������Ҳ�������ˮ����3��a��
��4�����������ȷų�������ƿ���������ݳ���
��5���μӷ�Ӧ�ĸ������������6.32g��
��������1��ֱ��д�����������ƣ�
��2��װ��AΪ�̹̼����͵ķ���װ�ã����Կ���������ȡ���������������ܹ�֧��ȼ�յ����ʣ������ô����ǵ�ľ������������壻���ݷ�Ӧ�����ͷ�Ӧ���״̬��ѡ����װ�ã�ѡ��������ռ�����ͨ���������ܶȺ�ˮ���ԣ�
��3���������շ��������ŵ���ѡ����ʵ�װ�ã�
��4������ˮ���ռ�����ʱӦ�õ����������ȵ�����ð��ʱ�����ռ���
��5�����������غ㶨�ɿ�����������ɵ�������������Ȼ����ݷ�Ӧ�Ļ�ѧ����ʽ��������������������������ص�������
���������⿼������������ȡԭ����װ�õ�ѡ��ֻҪ�����������ȡԭ��������װ��ѡ������ݣ��������ӭ�ж��⣮

��ϰ��ϵ�д�
 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�
�����Ŀ
 20����ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ���淶��ʵ�������ʵ��ɹ���ǰ�ᣬ��ش�
20����ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ���淶��ʵ�������ʵ��ɹ���ǰ�ᣬ��ش�