��Ŀ����
ij��ȤС��ͬѧ��ʵ�����Ʊ�������������������̽��ʵ�顣
��Ϊ̽�������������˫��ˮ�ֽ��ٶȵ�Ӱ�죬��������¶Ա�ʵ�飺
��3.0g 10%H2O2��Һ��1.0g MnO2���Ȼ��
��x g10% H2O2��Һ��1.0g CuO���Ȼ��
����ͬ�¶��£��Ƚ�����ʵ�����O2�Ŀ�����
���з�Ӧ�Ļ�ѧ�����ţ�����ʽ��____ ____������x��ֵӦΪ___ _____��
����̽����Ӱ��˫��ˮ�ֽ��ٶȵ�ij�����ء�ʵ�����ݼ�¼���£�
|
|
˫��ˮ������ |
˫��ˮ��Ũ�� |
MnO2������ |
��ͬʱ���ڲ���O2��� |
|
�� |
50.0g |
1% |
0.1g |
9 mL |
|
�� |
50.0g |
2% |
0.1g |
16 mL |
|
�� |
50.0g |
4% |
0.1g |
31 mL |
��ʵ���У�����O2�����װ����____ ____�����ţ���

ʵ����ۣ�����ͬ�����£�_______________��˫��ˮ�ֽ��Խ��.
������ͼװ�ý���ʵ�飬ͨ���Ƚ�___________Ҳ�ܴﵽʵ��Ŀ�ġ�

��2H2O2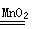 2H2O+O2�� 3.0
2H2O+O2�� 3.0
��C ˫��ˮŨ��Խ�� ��ͬʱ����ƽ������ֵ��С����������Ҳ�ɣ�
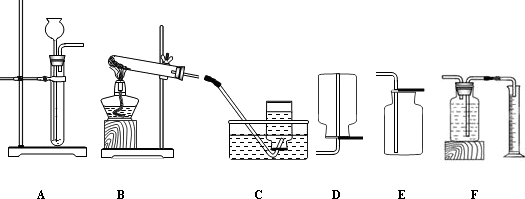
����������1���������ⷴӦ����ˮ����������Ӧ�Ļ�ѧ����ʽ2H2O2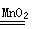 2H2O+O2�� ��ʵ��Ϊ�Ա�ʵ��������Ӧ����ͬ��xΪ3.0g
2H2O+O2�� ��ʵ��Ϊ�Ա�ʵ��������Ӧ����ͬ��xΪ3.0g
�ڲ���O2�����װ���Ҳ�ĵ���Ӧ���뼯��ƿ�ײ�����ѡC ���ɱ���֪˫��ˮŨ��Խ��˫��ˮ�ֽ��Խ�죬������ͬ��ʱ�������ͬ��˫��ˮ��������������ƽ������ֵ�Ĵ�С���бȽϣ���������Ҳ�ɣ�



 ij��ȤС��ͬѧ��ʵ�����Ʊ������������������������ʽ�������̽����
ij��ȤС��ͬѧ��ʵ�����Ʊ������������������������ʽ�������̽����

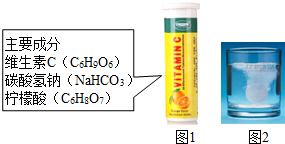 άC����Ƭ����Ҫ�ɷּ�ͼ1������ˮ�����������ݲ�������ͼ2����ij��ȤС��ͬѧ������ijɷֽ�������̽����
άC����Ƭ����Ҫ�ɷּ�ͼ1������ˮ�����������ݲ�������ͼ2����ij��ȤС��ͬѧ������ijɷֽ�������̽����