��Ŀ����
ʵ������һƿ���ʱ�䳤���������ƣ����е�һ�����Ѿ�ת������̼���ƣ���Ҫ�ø�ҩƷ����10%������������Һ��ȡ50.6g��ҩƷ������200gˮ�У�Ȼ�������ص���17.1%������������Һ������ȥ100g����������Һʱ��̼����ǡ����ȫ��Ӧ[Na2CO3+Ba��OH��2�TBaCO3��+2NaOH]����
��1��50.6g��ҩƷ��̼���Ƶ�������
��2����ȫ��Ӧ����Һ�����������Ƕ��٣�
��3����������Һ���ټ������gˮ�в������10%������������Һ��
��1��50.6g��ҩƷ��̼���Ƶ�������
��2����ȫ��Ӧ����Һ�����������Ƕ��٣�
��3����������Һ���ټ������gˮ�в������10%������������Һ��
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,�й��������������ļ���
ר�⣺�������������뻯ѧ����ʽ���ϵļ���
��������1��������������������������̼���������������ķ�Ӧ���̼���Ƶ��������ɣ�
��2����Һ���������Ƶ���������ԭ�������������Ƶ������뷴Ӧ���ɵ��������Ƶ�����֮�ͣ�����������������������ɵ��������Ƶ���������Һ���������ڻ��ǰ�����ʵ������������ɳ���������֮��ݴ���ɽ�ɣ�
��3�������Ӧ��Һ���������Ƶ�����������Ȼ�����ϡ��ǰ����Һ�����ʵ�����������н��
��2����Һ���������Ƶ���������ԭ�������������Ƶ������뷴Ӧ���ɵ��������Ƶ�����֮�ͣ�����������������������ɵ��������Ƶ���������Һ���������ڻ��ǰ�����ʵ������������ɳ���������֮��ݴ���ɽ�ɣ�
��3�������Ӧ��Һ���������Ƶ�����������Ȼ�����ϡ��ǰ����Һ�����ʵ�����������н��
����⣺��1����Ӧ����������������=100g��17.1%=17.1g
��50.6�˸�ҩƷ��̼���Ƶ�����Ϊx������NaOH������Ϊy������BaCO3������Ϊz��
Na2CO3+Ba��OH��2�TBaCO3��+2NaOH
106 171 197 80
x 17.1g z y
��
=
=
=
��
��ã�x=10.6g y=8g��z=19.7g
��2������������Һ�����ʵ�����=50.6g-10.6g+8g=48g
����������Һ������=50.6g+200g+100g-19.7g=330.9g
��ȫ��Ӧ����Һ�����������ǣ�
��100%=14.5%
��3�������ˮ�����=48g��10%-330.9g=149.1g
�𣺣�1��50.6g ��ҩƷ��̼���Ƶ�����Ϊ10.6g��
��2����ȫ��Ӧ����Һ������������14.5%��
��3����������Һ���ټ���149.1gˮ�������10%������������Һ��
��50.6�˸�ҩƷ��̼���Ƶ�����Ϊx������NaOH������Ϊy������BaCO3������Ϊz��
Na2CO3+Ba��OH��2�TBaCO3��+2NaOH
106 171 197 80
x 17.1g z y
��
| 106 |
| x |
| 171 |
| 17.1g |
| 197 |
| z |
| 80 |
| y |
��ã�x=10.6g y=8g��z=19.7g
��2������������Һ�����ʵ�����=50.6g-10.6g+8g=48g
����������Һ������=50.6g+200g+100g-19.7g=330.9g
��ȫ��Ӧ����Һ�����������ǣ�
| 48g |
| 330.9g |
��3�������ˮ�����=48g��10%-330.9g=149.1g
�𣺣�1��50.6g ��ҩƷ��̼���Ƶ�����Ϊ10.6g��
��2����ȫ��Ӧ����Һ������������14.5%��
��3����������Һ���ټ���149.1gˮ�������10%������������Һ��
������������һ����ѧ����ʽ�ļ����⣬����Ĺؼ����ܹ�����صķ�Ӧ�����գ����巴Ӧ��˼·��ϻ�ѧ����ʽ�ļ����ǽ���Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
�ؿ��к�����ߵĽ���Ԫ�غͺ�����ߵķǽ���Ԫ��������ʵĻ�ѧʽ�ǣ�������
| A��CaO |
| B��H2O |
| C��Al2O3 |
| D��Fe3O4 |
���з��Ų����к�����壬ֻ������������ǣ�������
| A��Fe |
| B��O2 |
| C��CO2 |
| D��2 O2 |
�ͷ����Ե��˵����ԭ������Ϊ238���˵����Ϊ92������������ӦΪ��������
| A��146 | B��92 |
| C��136 | D��238 |
����ʵ��������ܴﵽԤ��Ŀ���ǣ�������
| A����������ƽ��ȡ5.62g �Ȼ��ƹ��� |
| B����100����Һ����ȡ50.8����Һ�� |
| C����pH��ֽ��������pHֵΪ3 |
| D����5mLˮ��5mL�ƾ����Ƴ�10 mL�ƾ���Һ |
���б仯�У����ڻ�ѧ�仯���ǣ�������
| A���ƾ��ӷ� | B����˿���� |
| C��ľ��ȼ�� | D���������� |
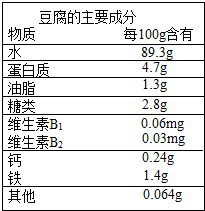 �ƶ������й��Ŵ���һ����Ҫ����������Զ�ľ����������Ե�������ɽˮĥ���Ƴɣ��Գ����ڸ��ۻ�������ӯ�ڶ�����������Ҫ�ɷ�����ͼ��ʾ��
�ƶ������й��Ŵ���һ����Ҫ����������Զ�ľ����������Ե�������ɽˮĥ���Ƴɣ��Գ����ڸ��ۻ�������ӯ�ڶ�����������Ҫ�ɷ�����ͼ��ʾ��