��Ŀ����
д������Ҫ��Ļ�ѧ����ʽ��
��1��ľ̿�ڿ�����ȼ��
��2��ϸ��˿��������ȼ��
��3�������ڿ�����ȼ��
��4��ˮ��ͨ�������·ֽ�
��5����������غͶ�������������
��1��ľ̿�ڿ�����ȼ��
C+O2
CO2
| ||
C+O2
CO2
| ||
��2��ϸ��˿��������ȼ��
3Fe+2O2
Fe3O4
| ||
3Fe+2O2
Fe3O4
| ||
��3�������ڿ�����ȼ��
4P+5O2
2P2O5
| ||
4P+5O2
2P2O5
| ||
��4��ˮ��ͨ�������·ֽ�
2H2O
2H2��+O2��
| ||
2H2O
2H2��+O2��
| ||
��5����������غͶ�������������
2KClO3
2KCl+3O2��
| ||
| �� |
2KClO3
2KCl+3O2��
��
| ||
| �� |
���������ȸ��ݷ�Ӧԭ���ҳ���Ӧ��������Ӧ���������ݻ�ѧ����ʽ����д���������������д���ɣ�
����⣺��1��ľ̿�ڿ�����ȼ�����ɶ�����̼����Ӧ�Ļ�ѧ����ʽΪ��C+O2
CO2�����C+O2
CO2��
��2����˿��������ȼ��������������������Ӧ�Ļ�ѧ����ʽΪ��3Fe+2O2
Fe3O4�����3Fe+2O2
Fe3O4��
��3�������ڿ�����ȼ���������������ף���Ӧ�Ļ�ѧ����ʽΪ��4P+5O2
2P2O5�����4P+5O2
2P2O5��
��4��ˮͨ��ֽ�������������������Ӧ�Ļ�ѧ����ʽΪ��2H2O
2H2��+O2�������2H2O
2H2��+O2����
��5��������ڶ������̵Ĵ������¼��������Ȼ��غ���������Ӧ�Ļ�ѧ����ʽΪ��2KClO3
2KCl+3O2�������2KClO3
2KCl+3O2����
| ||
| ||
��2����˿��������ȼ��������������������Ӧ�Ļ�ѧ����ʽΪ��3Fe+2O2
| ||
| ||
��3�������ڿ�����ȼ���������������ף���Ӧ�Ļ�ѧ����ʽΪ��4P+5O2
| ||
| ||
��4��ˮͨ��ֽ�������������������Ӧ�Ļ�ѧ����ʽΪ��2H2O
| ||
| ||
��5��������ڶ������̵Ĵ������¼��������Ȼ��غ���������Ӧ�Ļ�ѧ����ʽΪ��2KClO3
| ||
| �� |
| ||
| �� |
�����������ѶȲ�����ѧ�����ݷ�Ӧԭ����д��ѧ����ʽ����������ѧ����ʽ��д�������ֵĴ����в����Ͽ���ʵ�������������غ㶨�ɡ���д������������ŵȣ�

��ϰ��ϵ�д�
 ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д� �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�
�����Ŀ
Ԫ�����ڱ���ѧϰ���о���ѧ����Ҫ���ߣ���ͼ��Ԫ�����ڱ���һ���֣�
��1�����е�14��Ԫ���� ��
��2�������Ԫ�ص����ԭ�������� ��
��3�����ñ��е�Ԫ�ط�������ɵ�����д������Ҫ��Ļ�ѧ����ʽ���ж�����̼���ɵ������ֽⷴӦ�� �� ��
| �� ���� |
��A | O | ||||||
| 1 | 1H 1.008 |
��A | ��A | ��A | ��A | ��A | ��A | 2He 4..003 |
| 2 | 3Li 6.941 |
4Be 9.01 |
5B 10.811 |
6C 12.010 |
7N 14.0067 |
8O 15.999 |
9F 18.998 |
10Ne 20.179 |
| 3 | 11Na 22.989 |
12Mg 24.305 |
13Al 26.981 |
14Si 28.085 |
15P 30.973 |
16S 32��066 |
17Cl 35.452 |
18Ar 39.948 |
��2�������Ԫ�ص����ԭ��������
��3�����ñ��е�Ԫ�ط�������ɵ�����д������Ҫ��Ļ�ѧ����ʽ���ж�����̼���ɵ������ֽⷴӦ��
�±���Ԫ�����ڱ���һ���֣�
��1��16��Ԫ�ص�Ԫ�ط���Ϊ ����Ԫ�ص�ԭ�ӽṹʾ��ͼ���ң���X����ֵ= ��
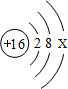
��2��8��Ԫ�غ�13��Ԫ����ɵĻ�����Ļ�ѧʽΪ ��
��3�����ñ��е�Ԫ�ط�������ɵķ�Ӧ��д������Ҫ��Ļ�ѧ����ʽ����ˮ���ɵĻ��Ϸ�Ӧ�� ��
| �� ���� |
IA | 0 | ||||||
| һ | 1H 1��008 |
��A | ��A | ��A | V A | ��A | ��A | 2He 4��003 |
| �� | 3Li 6��941 |
4Be 9��012 |
5B 10��81 |
6C 12��01 |
7N 14��01 |
8O 16��00 |
9F 19��00 |
10Ne 20��18 |
| �� | 11Na 22��99 |
12Mg 24��31 |
13Al 26��98 |
14Si 28��09 |
15P 30��97 |
16S 32��06 |
17Cl 35��45 |
18Ar 39��95 |
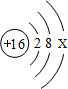
��2��8��Ԫ�غ�13��Ԫ����ɵĻ�����Ļ�ѧʽΪ
��3�����ñ��е�Ԫ�ط�������ɵķ�Ӧ��д������Ҫ��Ļ�ѧ����ʽ����ˮ���ɵĻ��Ϸ�Ӧ��