��Ŀ����
��2008?�ϸ�����ģ������һƿ��ǩ��ͼ��ʾ��Ũ���ᣬ����ݱ�ǩ�ϵ����ݻش����⣺| ���� �����500mL ��ѧʽ��HCl ��Է���������36.5 �ܶȣ�1.1g/cm3 ����������30% |
��2�����ø����Ƶ�ϡ�������ⶨij̼������Ʒ�Ĵ��ȣ�������Ԫ�أ���ȡһ������̼���Σ�R2C03����Ʒ�������е������õ�ϡ���������ٲ�������Ϊֹ������ȥ����73g�����ʲ������ᷴӦҲ������ˮ����Ȼ����ˣ�������2.8g����������Һ���ɣ��õ����崿����23.4g��
�ٷ�����Ӧ�Ļ�ѧ����ʽ��______�ڸ���Ʒ����Ҫ�ɷֻ�ѧʽ����______
��������֪�������μӷ�Ӧ�Ĺ�������������x���ı���ʽ______
�ܸ���Ʒ�Ĵ�����______
������Ӧ����Һ�м���31.6gˮ��ʱ������Һ�����ʵ���������Ϊ______��
���𰸡���������1���ɱ�ǩ��֪����Һ����������Ϊ30%����ˮϡ��Ϊ20%��ϡ���ᣬ��ˮǰ�������������䣻
��2��̼��������ų�������̼�����ݷ�Ӧ�е�����ȷ����Ӧ������ĩ�Ĵ��ȼ�������Һ����������������
����⣺��1�������ø�Ũ��������Ϊx
165g×20%=x?1.1g/cm3?30%
��֮�� x=100mL
�ʴ����ø�Ũ����100mL��
��2����̼������ϡ���������Ȼ��ˮ�Ͷ�����̼������̼���εĻ�ѧʽR2CO3���ɵ�֪Ԫ��R�Ļ��ϼ�Ϊ+2�ۣ�
�ʴ�R2CO3+2HCl=2RCl+CO2��+H2O��
����RԪ�ص����ԭ������Ϊm
R2CO3+2HCl=2RCl+CO2��+H2O
73 2m+71
73g×20% 23.4g
73����2m+71��=��73g×20%����23.4g
��֮�� m=23
�������ԭ�������ɲ��RԪ��Ϊ�ƣ�Na��
�ʴ�Na2CO3
��Na2CO3+2HCl=2NaCl+CO2��+H2O
106 73
x 73g×20%
106��73=x����73g×20%��
�ʴ�106��73=x����73g×20%��
�ܹ�����̼��������x=21.2g
��Ʒ�Ĵ���=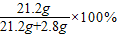 ��88.3%
��88.3%
�ʴ�88.3%
���跴Ӧ���ɶ�����̼����Ϊy
Na2CO3+2HCl=2NaCl+CO2��+H2O
73 44
73g×20% y
73��44=��73g×20%����y y=8.8g
����31.6gˮ��ʱ������Һ�����ʵ���������=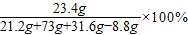 =20%
=20%
�ʴ�20%
���������������غ㶨�ɣ�����31.6gˮ��������Һ������=21.2g+73g+31.6g-8.8g=117g��
��2��̼��������ų�������̼�����ݷ�Ӧ�е�����ȷ����Ӧ������ĩ�Ĵ��ȼ�������Һ����������������
����⣺��1�������ø�Ũ��������Ϊx
165g×20%=x?1.1g/cm3?30%
��֮�� x=100mL
�ʴ����ø�Ũ����100mL��
��2����̼������ϡ���������Ȼ��ˮ�Ͷ�����̼������̼���εĻ�ѧʽR2CO3���ɵ�֪Ԫ��R�Ļ��ϼ�Ϊ+2�ۣ�
�ʴ�R2CO3+2HCl=2RCl+CO2��+H2O��
����RԪ�ص����ԭ������Ϊm
R2CO3+2HCl=2RCl+CO2��+H2O
73 2m+71
73g×20% 23.4g
73����2m+71��=��73g×20%����23.4g
��֮�� m=23
�������ԭ�������ɲ��RԪ��Ϊ�ƣ�Na��
�ʴ�Na2CO3
��Na2CO3+2HCl=2NaCl+CO2��+H2O
106 73
x 73g×20%
106��73=x����73g×20%��
�ʴ�106��73=x����73g×20%��
�ܹ�����̼��������x=21.2g
��Ʒ�Ĵ���=
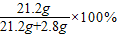 ��88.3%
��88.3%�ʴ�88.3%
���跴Ӧ���ɶ�����̼����Ϊy
Na2CO3+2HCl=2NaCl+CO2��+H2O
73 44
73g×20% y
73��44=��73g×20%����y y=8.8g
����31.6gˮ��ʱ������Һ�����ʵ���������=
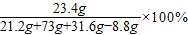 =20%
=20%�ʴ�20%
���������������غ㶨�ɣ�����31.6gˮ��������Һ������=21.2g+73g+31.6g-8.8g=117g��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��2008?�ϸ�����ģ��������ͼij�̷۰�װ�ϵIJ�������˵�����ش����⣺
��1�����̷��п�Ϊ�����ṩ����Ԫ����______����������Ҫ��______������Ӫ����֮һ������ʽ���ڣ�
��2��ÿ��֬���������ڷֽ�ƽ���ų�38.9KJ����������ijͬѧÿ���100gţ�̣���ţ��������֬���ṩ���������Ϊ______
��1�����̷��п�Ϊ�����ṩ����Ԫ����______����������Ҫ��______������Ӫ����֮һ������ʽ���ڣ�
��2��ÿ��֬���������ڷֽ�ƽ���ų�38.9KJ����������ijͬѧÿ���100gţ�̣���ţ��������֬���ṩ���������Ϊ______
| ��������480g Ӫ���ɷ֣� ֬����20%-25% �����ʣ�25% п����20mg �ף���500mg �ƣ���400mg ������30mg |
 ��
��
