题目内容
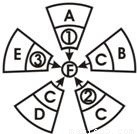
某同学学习过程中发现物质的制备有不同的方法,他用“旋转的风车”归纳了某镁盐F的制备方法(如下图)。其中物质①、②、③依次分别是CuCl2、MgCO3、MgSO4,物质A、B、C、D、E依次分别属于单质、氧化物、酸、碱、盐。“风车”中每片“叶轮”上相邻的两种物质相互反应都能生成物质F。则:
(1)物质A、D的化学式分别是___________、___________;
(2)物质C与②反应的主要现象是_______________________;
(3)物质B与C反应的化学方程式为________________________。
(2)物质C与②反应的主要现象是_______________________;
(3)物质B与C反应的化学方程式为________________________。
(1)Mg;Mg(OH)2
(2)产生大量无色气泡
(3)MgO+2HCl==MgCl2+H2O
(2)产生大量无色气泡
(3)MgO+2HCl==MgCl2+H2O

练习册系列答案
 天天向上一本好卷系列答案
天天向上一本好卷系列答案 小学生10分钟应用题系列答案
小学生10分钟应用题系列答案
相关题目
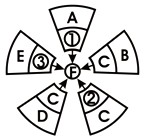
 23、某同学学习过程中发现物质的制备有不同的方法,他用“旋转的风车”归纳了某镁盐 F 的制备方法(如右图).其中物质①、②、③依次分别是CuCl2、MgCO3、MgSO4,物质A、B、C、D、E依次分别属于单质、氧化物、酸、碱、盐.“风车”中每片“叶轮”上相邻的两种物质相互反应都能生成物质F.则:
23、某同学学习过程中发现物质的制备有不同的方法,他用“旋转的风车”归纳了某镁盐 F 的制备方法(如右图).其中物质①、②、③依次分别是CuCl2、MgCO3、MgSO4,物质A、B、C、D、E依次分别属于单质、氧化物、酸、碱、盐.“风车”中每片“叶轮”上相邻的两种物质相互反应都能生成物质F.则: