��Ŀ����
 11��ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ������
11��ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ��������1����Щ��ׯ���ȡ�õ���ˮ���������ˮ��Ӳˮ������ˮ�����õ�������
����ˮ
���ⶨ����ˮ�����ȿ���pH��ֽ
����2����Щ����ȡ���ǵĿ�ˮ��������ˮ����ͬѧ������ѧ��֪ʶ�����ǵĿ�ˮ����ͼ��ʾ�ļ���ˮ�����о���������С��ʯ��ʯӢɳ��������
����
����3���������ˮӲ�ȴ��߿�ˮ�в�ԭ������࣬�����Բ�ȡ
�������
������������Ӳ�Ⱥ�ɱ��ԭ�����4������ط���Һ����ɱ��������ʵʩ������˹����꣮Һ����ɱ������ԭ����
�������Ʋ��б����̬ʱ���մ������ȣ�ʹ�Ʋ���ˮ���������С��Σ�
���������÷���ˮ���Լ���ˮ����Ӳ����pH��ֽ���Բⶨ����ˮ�����ȣ����˿��Գ�ȥ������ˮ�����ʣ�������мȿ��Խ���ˮ��Ӳ�ȣ��ֿ���ɱ��������Һ��������ɱ�����ʱ�ܹ�������������Լ��ˮ�����츣�����
����⣺��1���÷���ˮ���Լ���ˮ����Ӳ����pH��ֽ���Բⶨ����ˮ�����ȣ��������ˮ��pH��ֽ��
��2��С��ʯ��ʯӢɳ�������ǹ��ˣ�������ˣ�
��3��������мȿ��Խ���ˮ��Ӳ�ȣ��ֿ���ɱ�����������������У�
��4��Һ����ɱ������ԭ���ǣ��������Ʋ��б����̬ʱ���մ������ȣ�ʹ�Ʋ���ˮ���������С��Σ�
�ʴ�Ϊ����1������ˮ��pH��ֽ��
��2�����ˣ�
��3���������
��4���������Ʋ��б����̬ʱ���մ������ȣ�ʹ�Ʋ���ˮ���������С��Σ�
��2��С��ʯ��ʯӢɳ�������ǹ��ˣ�������ˣ�
��3��������мȿ��Խ���ˮ��Ӳ�ȣ��ֿ���ɱ�����������������У�
��4��Һ����ɱ������ԭ���ǣ��������Ʋ��б����̬ʱ���մ������ȣ�ʹ�Ʋ���ˮ���������С��Σ�
�ʴ�Ϊ����1������ˮ��pH��ֽ��
��2�����ˣ�
��3���������
��4���������Ʋ��б����̬ʱ���մ������ȣ�ʹ�Ʋ���ˮ���������С��Σ�
�����������Ҫ������⾻��ˮ�����֪ʶ��ֻ���������ܶ���ط��������������ȷ���жϣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ


 47��ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ������
47��ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ������ ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ������
ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ������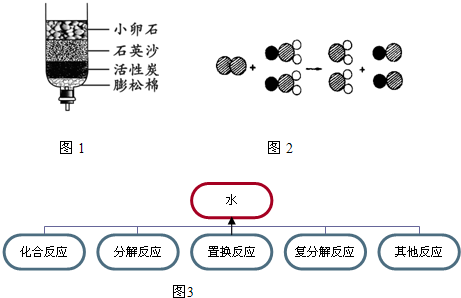
 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�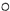 ��ʾ��ԭ�ӣ�����д����Ӧ�Ļ�ѧ����ʽ
��ʾ��ԭ�ӣ�����д����Ӧ�Ļ�ѧ����ʽ