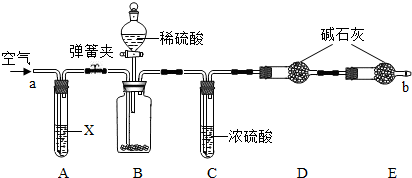
��Ŀ����
�á������Ƽ���ƵõĴ����г������������Ȼ��ƣ�Ϊ�ⶨij������Ʒ��̼���Ƶ�����������С��ͬѧ��ȡ����Ʒ5.6 g���뵽ʢ��100gϡ������ձ��У���ȫ��Ӧ��Ƶ��ձ��е���Һ����Ϊ103.4g������Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+2HCl=2NaCl+CO2��+H2O��
��
��1����ȫ��Ӧ�����ɶ�����̼������Ϊ________ g��
��2���ô�����Ʒ��̼���Ƶ������������������ȷ��0.1%��
�⣺��1�����������ض��ɼ������֪�����ɵĶ�����̼������Ϊ��5.6 g+100g-103.4g=2.2g��
��2����̼���Ƶ�����ΪX
Na2CO3+2HCl=2NaCl+CO2��+H2O
106 44
X 2.2g
 ��ã�X=5.3g
��ã�X=5.3g
�ô�����Ʒ��̼���Ƶ���������Ϊ��
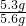 ��100%=94.6%
��100%=94.6%
�𣺣�1��2.2����2���ô�����Ʒ��̼���Ƶ���������94.6%��
��������1�����������غ㶨�ɣ���Ӧǰ���ձ������������ٵ�����Ϊ������̼��������
��2���ɶ�����̼������������̼���������ᷴӦ�ķ���ʽ�����̼���Ƶ��������Ϳ�������ô�����Ʒ��̼���Ƶ�����������
���������������غ㶨�ɷ�����Ӧǰ�������仯����÷�Ӧ���ɶ�����̼���������ɴ��������û���֪ʶ������ķ���������
��2����̼���Ƶ�����ΪX
Na2CO3+2HCl=2NaCl+CO2��+H2O
106 44
X 2.2g
 ��ã�X=5.3g
��ã�X=5.3g�ô�����Ʒ��̼���Ƶ���������Ϊ��
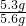 ��100%=94.6%
��100%=94.6%�𣺣�1��2.2����2���ô�����Ʒ��̼���Ƶ���������94.6%��
��������1�����������غ㶨�ɣ���Ӧǰ���ձ������������ٵ�����Ϊ������̼��������
��2���ɶ�����̼������������̼���������ᷴӦ�ķ���ʽ�����̼���Ƶ��������Ϳ�������ô�����Ʒ��̼���Ƶ�����������
���������������غ㶨�ɷ�����Ӧǰ�������仯����÷�Ӧ���ɶ�����̼���������ɴ��������û���֪ʶ������ķ���������

��ϰ��ϵ�д�
�����Ŀ