��Ŀ����
18���������ƣ�Na2O2����һ�ֻ�ѧ���ʻ��õĵ���ɫ���壬��ͨ��״�����ܸ��������ʷ�����ѧ��Ӧ�����磺2Na2O2+2CO2�T2Na2CO3+O2����2Na2O2+4HCl�T4NaCl+2H2O+O2����2Na2O2+2H2O�T4NaOH+O2��
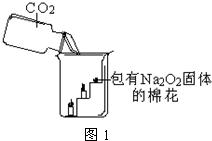
Ϊ����֤CO2��Na2O2��Ӧ������������O2���������ͼ��ʵ��װ�ã��Իش�

��1�����A��Bװ�õ�Ŀ���Ǿ��������CO2�����Т�Aװ�õľ���������
��Bװ�õ�������
���罫A��B��ƿ����ʢ��Һ�廥����ֱ�ӵĺ����
��2��Cװ�õľ���������
��3��Ϊ��ȷ֤CO2��Na2O2��Ӧ���������������������Ҫ���õ���֤ʵ�������
��4����ⶨʢ��Na2O2�ķ�Ӧ����������Ӧ������������ag�������������������Ϊ
Ϊ����֤CO2��Na2O2��Ӧ������������O2���������ͼ��ʵ��װ�ã��Իش�

��1�����A��Bװ�õ�Ŀ���Ǿ��������CO2�����Т�Aװ�õľ���������
��ȥCO2���������Ȼ�������
����ƿ��Һ�����������NaHCO3��Һ
����������Ӧ�Ļ�ѧ����ʽ��NaHCO3+HCl�TNaCl+H2O+CO2��
����Bװ�õ�������
��ȥAƿ�е�����CO2�е�ˮ����
����ƿ��Һ��Ӧ��Ũ����
�����罫A��B��ƿ����ʢ��Һ�廥����ֱ�ӵĺ����
��Bƿ�е�����CO2����ˮ����
����2��Cװ�õľ���������
��ȥ��Ӧ�������в����CO2
����ƿ��Һ��Ӧ��NaOH��Һ
����������Ӧ�Ļ�ѧ����ʽ��2NaOH+CO2�TNa2CO3+H2O
����3��Ϊ��ȷ֤CO2��Na2O2��Ӧ���������������������Ҫ���õ���֤ʵ�������
�������ǵ�ľ�����ڵ��ܿڣ�ľ����ȼ
����4����ⶨʢ��Na2O2�ķ�Ӧ����������Ӧ������������ag�������������������Ϊ
4a/7
���ڱ�״���£������ܶ�Ϊ1.43g/L����������ˮ��Һ�壩���ܽ�������Ժ��Բ��ƣ���ʵ���п����ռ������������Ϊ0.4a
L��������Ϊ����֤CO2��Na2O2��Ӧ������������O2������ΪNa2O2��ˮ��HCl���ܷ�Ӧ�����������������ͨ��Na2O2ǰ�����Ƚ�ˮ��HCl����ȥ�����ֱ�ͨ��Ũ����ͱ���NaHCO3��Һ������ͨ������NaHCO3��Һʱ�����ˮ����������ȳ�ȥHCl�����ȥˮ�������罫A��B��ƿ����ʢ��Һ�廥����ֱ�ӵĺ���Ǵ�Bƿ�е�����CO2����ˮ�����������ļ��鷽�����ô����ǵ�ľ�����Ƿ�ȼ��Ϊ��ȷ֤CO2��Na2O2��Ӧ�����������������轫�����ǵ�ľ�����ڵ��ܿڣ�����2Na2O2+2CO2�T2Na2CO3+O2����֪����Ӧװ��ʵ�����ӵ���Ϊ88-32=56���ָ������������ԭ������Ϊ32������������������ΪX����������ã�56��32=a��x�����X=4a/7g������������������ݹ�ʽ�����=$\frac{����}{�ܶ�}$����������������Ϊ0.4aL��
����⣺Ϊ����֤CO2��Na2O2��Ӧ������������O2������ΪNa2O2��ˮ��HCl���ܷ�Ӧ�����������������ͨ��Na2O2ǰ�����Ƚ�ˮ��HCl����ȥ�����ֱ�ͨ��Ũ����ͱ���NaHCO3��Һ������ͨ������NaHCO3��Һʱ�����ˮ����������ȳ�ȥHCl�����ȥˮ�������罫A��B��ƿ����ʢ��Һ�廥����ֱ�ӵĺ���Ǵ�Bƿ�е�����CO2����ˮ�����������ļ��鷽�����ô����ǵ�ľ�����Ƿ�ȼ��Ϊ��ȷ֤CO2��Na2O2��Ӧ�����������������轫�����ǵ�ľ�����ڵ��ܿڣ�
�ʴ�Ϊ����1���ٳ�ȥCO2���������Ȼ������履��NaHCO3��ҺNaHCO3+HCl�TNaCl+H2O+CO2�����ڳ�ȥAƿ�е�����CO2�е�ˮ����Ũ�������ͨ������NaHCO3��Һʱ�����ˮ����������ȳ�ȥHCl�����ȥˮ�������۴�Bƿ�е�����CO2����ˮ������2����ȥ��Ӧ�������в����CO2NaOH��Һ2NaOH+CO2�TNa2CO3+H2O��3���������ǵ�ľ�����ڵ��ܿڣ�ľ����ȼ��4��4a/70.4a
�ʴ�Ϊ����1���ٳ�ȥCO2���������Ȼ������履��NaHCO3��ҺNaHCO3+HCl�TNaCl+H2O+CO2�����ڳ�ȥAƿ�е�����CO2�е�ˮ����Ũ�������ͨ������NaHCO3��Һʱ�����ˮ����������ȳ�ȥHCl�����ȥˮ�������۴�Bƿ�е�����CO2����ˮ������2����ȥ��Ӧ�������в����CO2NaOH��Һ2NaOH+CO2�TNa2CO3+H2O��3���������ǵ�ľ�����ڵ��ܿڣ�ľ����ȼ��4��4a/70.4a
�������ۺ�ʵ���ǽ��������������������������Ʊ������ʵļ��顢�����ʡ���ѧ��Ӧ��������ۺϵ�һ���ڽ���ʱע��Ū���ʵ���˼·����ʵ���Ŀ����ʲô��Ŀ�������ʵ�ֵģ���Ҫ������Щ������ʵ������в�������Щ������Щ�������ܵõ���Щ���۵ȣ�

��ϰ��ϵ�д�
�����Ŀ


 23��������ߺ�DZˮͧ�п��ù������ƣ�Na2O2����Ϊ����������������ȤС��ͬѧΧ�ƹ������ƽ��е�һϵ��̽����������뵽���У�
23��������ߺ�DZˮͧ�п��ù������ƣ�Na2O2����Ϊ����������������ȤС��ͬѧΧ�ƹ������ƽ��е�һϵ��̽����������뵽���У�
