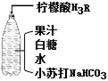
��Ŀ����
����ͼ�䷽������һƿ�����������ˮ��

����ͼ����Ϣ�ش�
��1��С�մ��к��еĽ���Ԫ�ص�������____________��
��2����ˮ�к����������ʵĻ�ѧʽ��______________-��
��3����ƿ�Dz������壬˵�������ܽ����__________�йأ�
��4��������ˮʱ������Ӧ�Ļ�ѧ����ʽΪ��3NaHCO3+H3R==Na3R+ 3H2O+3X������X�Ļ�ѧʽ��____��
��5�����ǵ���Ҫ�ɷ���C12H22O11������̼��������Ԫ�ص���������___________��
��1��С�մ��к��еĽ���Ԫ�ص�������____________��
��2����ˮ�к����������ʵĻ�ѧʽ��______________-��
��3����ƿ�Dz������壬˵�������ܽ����__________�йأ�
��4��������ˮʱ������Ӧ�Ļ�ѧ����ʽΪ��3NaHCO3+H3R==Na3R+ 3H2O+3X������X�Ļ�ѧʽ��____��
��5�����ǵ���Ҫ�ɷ���C12H22O11������̼��������Ԫ�ص���������___________��
��1����
��2��H2O
��3��ѹǿ
��4��CO2
��5��72��11
��2��H2O
��3��ѹǿ
��4��CO2
��5��72��11

��ϰ��ϵ�д�
�����Ŀ