��Ŀ����
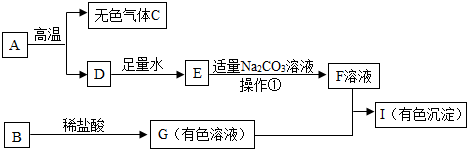
 23��֪ʶ����ͼ������֪ʶ����ںϣ����п�ͼ�dz��л�ѧ���ֳ�������֮���ת����ϵ������A��һ�ֽ������ϵ���Ҫ�ɷ֣�B�����������ش�
23��֪ʶ����ͼ������֪ʶ����ںϣ����п�ͼ�dz��л�ѧ���ֳ�������֮���ת����ϵ������A��һ�ֽ������ϵ���Ҫ�ɷ֣�B�����������ش���1��д���������ʵĻ�ѧʽ��A��
CaCO3
C��CO2
����2���õ�F��Һ�IJ����ٵ�����Ϊ��
����
����3����I�Ǻ��ɫ��������G+F��I�Ļ�ѧ����ʽΪ��
3NaOH+FeCl3=Fe��OH��3��+3NaCl
����4����I����ɫ��������B+ϡ�����G�Ļ�ѧ����ʽΪ��
CuO+2HCl=CuCl2+H2O
����������1������A�ǽ������ϵ���Ҫ�ɷ֣�A�ڸ������ֻ�������ɫ����C��D������A����̼��ƣ�����C���Ƕ�����̼��D���������ƣ������ƺ�ˮ�����������ƣ�E�����������ƣ��������ƺ�̼��������̼��Ƴ������������ƣ�����F�����������ƣ�
��2��������ν������ԵĹ������Һ�з�����з�����
��3�����ɫ�ij�������������������Ȼ���Ƴ�����ʽ��
��4������I����ɫ����������ͭ���ӵij������з�����
��2��������ν������ԵĹ������Һ�з�����з�����
��3�����ɫ�ij�������������������Ȼ���Ƴ�����ʽ��
��4������I����ɫ����������ͭ���ӵij������з�����
����⣺��1��A�ǽ������ϵ���Ҫ�ɷ֣�A�ڸ������ֻ�������ɫ����C��D������A����̼��ƣ�����C���Ƕ�����̼��D���������ƣ������ƺ�ˮ�����������ƣ�E�����������ƣ��������ƺ�̼��������̼��Ƴ������������ƣ�����F�����������ƣ��ʴ�Ϊ��CaCO3��CO2��
��2�������ǽ������ԵĹ������Һ�з����һ�ַ������������ƺ�̼���Ʒ�Ӧ����̼��Ƴ������������ƣ�Ҫ�õ�F��Һ����Ҫ���ˣ��ʴ�Ϊ�����ˣ�
��3�����ɫ�ij�������������������F���������ƣ�G���Ȼ�������G+F��I�Ļ�ѧ����ʽΪ3NaOH+FeCl3=Fe��OH��3��+3NaCl��
��4��I����ɫ������G�����Ȼ�ͭ����B+ϡ�����G�Ļ�ѧ����ʽΪ��2NaOH+CuCl2�TCu��OH��2��+2NaCl��
�ʴ�Ϊ����1��A��CaCO3 C��CO2
��2�����ˣ�
��3��3NaOH+FeCl3=Fe��OH��3��+3NaCl
��4��CuO+2HCl=CuCl2+H2O
��2�������ǽ������ԵĹ������Һ�з����һ�ַ������������ƺ�̼���Ʒ�Ӧ����̼��Ƴ������������ƣ�Ҫ�õ�F��Һ����Ҫ���ˣ��ʴ�Ϊ�����ˣ�
��3�����ɫ�ij�������������������F���������ƣ�G���Ȼ�������G+F��I�Ļ�ѧ����ʽΪ3NaOH+FeCl3=Fe��OH��3��+3NaCl��
��4��I����ɫ������G�����Ȼ�ͭ����B+ϡ�����G�Ļ�ѧ����ʽΪ��2NaOH+CuCl2�TCu��OH��2��+2NaCl��
�ʴ�Ϊ����1��A��CaCO3 C��CO2
��2�����ˣ�
��3��3NaOH+FeCl3=Fe��OH��3��+3NaCl
��4��CuO+2HCl=CuCl2+H2O
�������ڽ������ʱ���Ƚ������������������Ƴ���Ȼ���ٸ����Ƴ������ʺ����е�������Ӧ�Ƴ�ʣ������ʣ�����ٽ�����֤���ɣ�

��ϰ��ϵ�д�
�����Ŀ