��Ŀ����
��ȡNaCl��BaCl2�Ĺ�������32.5 g������100 g����ˮ����ȫ�ܽ����û����Һ����μ�����������Ϊ10%��Na2SO4��Һ����Ӧ����BaSO4�������������������Na2SO4��Һ��������ϵ����ͼ��ʾ���Իش��������⣺
(��ʾ��BaCl2+Na2SO4====BaSO4��+2NaCl)

(1)��ȫ��Ӧ������BaSO4����______________g��
(2)ǡ����ȫ��Ӧʱ����Na2SO4��Һ��������____________________g��
(3)ǡ����ȫ��Ӧʱ������Һ�����ʵ����������Ƕ��٣�(��ȷ��0.1%)
���⿼����ͼ����л�ѧ����ʽ�����������
(1)������ͼʾ��֪����ȫ��Ӧ������BaSO4����������Ϊ23.3 g��
(2)����BaSO4����������������Ƶ�������������������Һ�����ʵ������������������������Һ��������
(3)ǡ����ȫ��Ӧ����Һ���������Ȼ��ƣ����ݳ���������������÷�Ӧ���ɵ��Ȼ��Ƶ�����������ԭ�������Ȼ��Ƶ�������Ϊ��Ӧ����Һ�����ʵ���������һ�������ǡ����ȫ��Ӧʱ������Һ�����ʵ�����������
�𰸣�(1)23.3��(2)142
(3)�⣺��BaCl2������Ϊx����Ӧ���ɵ�NaCl������Ϊy��
BaCl2+Na2SO4====BaSO4��+2NaCl
208 233 117
x 23.3 g y
 =
= =
=
x=20.8 g��y=11.7 g
ǡ����ȫ��Ӧʱ����Һ��NaCl������Ϊ
11.7 g+(32.5 g-20.8 g)=23.4 g
NaCl��Һ���ʵ���������Ϊ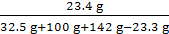 ��100%=9.3%
��100%=9.3%
��(��)

��һ��������Ba(OH)2��Һ��Na2CO3��Һ���ǡ����ȫ��Ӧ����Ӧ��Ļ�����м���ϡ���ᣬ�����������������ϡ���������Ĺ�ϵ������ͼ��ʾ������˵������ȷ���ǣ� ��

A��N ��ʱ��������Һ��pH=7
B��Q��ʱ��������Һ�е�����ֻ����BaCl2
C��O��P�η�����Ӧ�Ļ�ѧ����ʽΪNaOH+HCl=NaCl+H2O
D��P��Q�η�����Ӧ�Ļ�ѧ����ʽΪBa(OH)2+2HCl=BaCl2+2H2O
| M������/ g | S������/ | M2S������/ g | |
| �� | 6.0 | 2.5 | 7.5 |
| �� | 7.0 | 1.5 | 7.5 |
 ��������ʳ��
��������ʳ��


 g
g