��Ŀ����
ijͬѧ����±���ʾʵ�飬̽������ͭ��H2O2�ֽ����ʵ�Ӱ�죮
| ���� | װ�� | ���� |
| 1��ȡһС��ͭ˿��������Ͳ�Σ��̶���ͭ˿�ϣ� |  | |
| 2���ֱ���ٺ͢���ע��15mL��30%��H2O2��������ʢ����ˮ�Ģ��У� |  | Լ1min��ɹ۲쵽�� �͢��ж����������ݲ��� |
| 3����ͭ˿������У� |  | �����д������ݲ�����Լ5min���������ݲ�������ʱ���� ��Ȼ���������ݲ��� |
��1����������ʵ���Ŀ����______��
��2����������ʵ�飬���Եó��Ľ�����______��
��3����д���Թܢ��з�Ӧ�ķ���ʽ______��
��4������ñ�ʵ��̽��ͭ�Dz���H2O2�ֽ�Ĵ�������Ҫ������ʵ�鷽�����в��䣬���б�Ҫ����______��
A������ʵ��ǰͭ˿������������������������B����ʵ����ͭ˿���������
C���������м���������̡�����������������D����ʵ����ͭ˿������AgNO3��Һ��
��5�����Ҫ����300g5%��H2O2��Һ����______g30%Ũ��H2O2��Һ��
�⣺��1�������ٺ������ڼ���Ĺ��������Ũ����ͬ��������������û�м���ͭ������������������������Ƕ������ã�
��2��������ʵ�飬�ó�ͭ���������ܼӿ�H2O2�ֽ�����ʣ�
��3����������ֽ�������ˮ����������Ӧ�ķ���ʽ�ǣ�2H2O2=2H2O+O2����
��4���ɴ����Ķ����֪�������ڷ�Ӧǰ�������ͻ�ѧ���ʲ��䣬Ҫ̽��ͭ�Dz���H2O2�ֽ�Ĵ�������Ҫ������ʵ�鷽�����в��䣬���б�Ҫ���ǣ�����ʵ��ǰͭ˿������������ʵ����ͭ˿��������أ��Աȷ�Ӧǰ����������Ƿ�仯����ʵ����ͭ˿������AgNO3��Һ�У�̽��ͭ�������Ƿ�ı䣻
��5������Ҫ30%Ũ��H2O2��Һ������ΪX����
300g��5%=X��30% ��ã�X=50g
�ʴ�Ϊ����1���͢����Աȣ���2��ͭ�ܼӿ�H2O2�ֽ�����ʣ���3��2H2O2=2H2O+O2������4��ABD����5��50g��
��������1��ͨ���ԱȲ��ܹ������ͭ�������ã��ɴ˿��Եó������ڵ����ã�
��2����������ʵ�飬����ͭ�����ã�
��3�����ݷ�Ӧд����Ӧ�ķ���ʽ��
��4�����ݴ������ص㣺�ڷ�Ӧǰ�������ͻ�ѧ���ʲ��䣬�������б�Ҫ�IJ�����
��5������������������ԭ�����м��㣮
��������������ʵ��̽���⣬����ʱҪ����˼·����̽�����ʵ�����ʱ����õķ��������öԱȷ��������ʵ����ʣ�
��2��������ʵ�飬�ó�ͭ���������ܼӿ�H2O2�ֽ�����ʣ�
��3����������ֽ�������ˮ����������Ӧ�ķ���ʽ�ǣ�2H2O2=2H2O+O2����
��4���ɴ����Ķ����֪�������ڷ�Ӧǰ�������ͻ�ѧ���ʲ��䣬Ҫ̽��ͭ�Dz���H2O2�ֽ�Ĵ�������Ҫ������ʵ�鷽�����в��䣬���б�Ҫ���ǣ�����ʵ��ǰͭ˿������������ʵ����ͭ˿��������أ��Աȷ�Ӧǰ����������Ƿ�仯����ʵ����ͭ˿������AgNO3��Һ�У�̽��ͭ�������Ƿ�ı䣻
��5������Ҫ30%Ũ��H2O2��Һ������ΪX����
300g��5%=X��30% ��ã�X=50g
�ʴ�Ϊ����1���͢����Աȣ���2��ͭ�ܼӿ�H2O2�ֽ�����ʣ���3��2H2O2=2H2O+O2������4��ABD����5��50g��
��������1��ͨ���ԱȲ��ܹ������ͭ�������ã��ɴ˿��Եó������ڵ����ã�
��2����������ʵ�飬����ͭ�����ã�
��3�����ݷ�Ӧд����Ӧ�ķ���ʽ��
��4�����ݴ������ص㣺�ڷ�Ӧǰ�������ͻ�ѧ���ʲ��䣬�������б�Ҫ�IJ�����
��5������������������ԭ�����м��㣮
��������������ʵ��̽���⣬����ʱҪ����˼·����̽�����ʵ�����ʱ����õķ��������öԱȷ��������ʵ����ʣ�

��ϰ��ϵ�д�
 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�
�����Ŀ
 ij��ѧ��ȤС���ͬѧ���֣����ͷ��ܹ�ʹ������������������Ϊ���ͷ۲�����CO2���£����ͷ۵���Ҫ�ɷ�����̼�����ƣ�NaHCO3�����׳�С�մ����Ƕ�̼�����Ƶ����ʽ�����̽����
ij��ѧ��ȤС���ͬѧ���֣����ͷ��ܹ�ʹ������������������Ϊ���ͷ۲�����CO2���£����ͷ۵���Ҫ�ɷ�����̼�����ƣ�NaHCO3�����׳�С�մ����Ƕ�̼�����Ƶ����ʽ�����̽����ʵ��һ��̽��̼��������Һ�������
��pH��ֽ���̼��������Һ��pHΪ10���ɴ˿�֪̼��������Һ��
ʵ�����̽��̼�����Ƶ����ȶ���
���������ϡ�̼�������������ֽ⣬����ˮ��������̼�����һ�ֳ����Ĺ������ʣ�
������ʵ�顿Ϊ��֤̼����������ʱ��ֽ⣬��ȤС���ͬѧȡһ��������̼�����Ʒŵ�ͭƬ�ϼ��ȣ���ͼ��ʾ��
��1������һ��ʱ��۲쵽�ձ��ڱ���
��2����ּ��Ⱥ��ձ�Ѹ�ٵ�ת���������������ij���ʯ��ˮ�����۲쵽ʯ��ˮ����ǣ�д���÷�Ӧ�Ļ�ѧ����ʽ��
��3����ȤС���ͬѧ��Ϊ��ּ��Ⱥ�Ĺ�����������NaOH��Na2CO3��
�����Dz����������
�������ʵ����鷴Ӧ��Ĺ��������NaOH����Na2CO3���������±���
| ʵ �� �� �� | Ԥ��ʵ������ | �� �� |
| ���������Na2CO3��������NaOH�� |
NaHCO3���ȷ����仯�Ļ�ѧ����ʽΪ
 26��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����
26��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����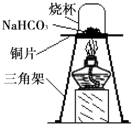 ij��ѧ��ȤС���ͬѧ���֣����ͷ��ܹ�ʹ������������������Ϊ���ͷ۲�����CO2���£����ͷ۵���Ҫ�ɷ�����̼�����ƣ�NaHCO3�����׳�С�մ����Ƕ�̼�����Ƶ����ʽ�����̽����
ij��ѧ��ȤС���ͬѧ���֣����ͷ��ܹ�ʹ������������������Ϊ���ͷ۲�����CO2���£����ͷ۵���Ҫ�ɷ�����̼�����ƣ�NaHCO3�����׳�С�մ����Ƕ�̼�����Ƶ����ʽ�����̽����
