��Ŀ����
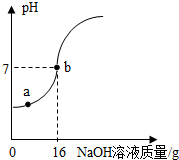 ��2013?̫ԭ��ʵ�������ⶨһƿ��ǩ�����ϡH2SO4������������������ȡ10gϡ������Ʒ����5%��NaOH��Һ��μӵ���Ʒ�У��ӱ߽��裬����NaOH��Һ�IJ��ϼ��룬��ҺpH�ı仯��ͼ��ʾ���Իش�
��2013?̫ԭ��ʵ�������ⶨһƿ��ǩ�����ϡH2SO4������������������ȡ10gϡ������Ʒ����5%��NaOH��Һ��μӵ���Ʒ�У��ӱ߽��裬����NaOH��Һ�IJ��ϼ��룬��ҺpH�ı仯��ͼ��ʾ���Իش���1��a����Һ�к��е�������
Na+��H+��SO42-
Na+��H+��SO42-
����2����pH=7ʱ������NaOH��Һ�е�NaOH������Ϊ
0.8
0.8
g����3������ϡH2SO4����������������
��������1��������Һ��pH��ϡH2SO4��NaOH��Һ�ķ�Ӧ������Һ�д��ڵ����ӣ�
��2�������������������ļ��㹫ʽ���㣻
��3������ϡH2SO4��NaOH��Һ�ķ�Ӧ�ķ���ʽ����NaOH���������ϡH2SO4������ϡH2SO4����������������
�����ϡH2SO4����������������
��2�������������������ļ��㹫ʽ���㣻
��3������ϡH2SO4��NaOH��Һ�ķ�Ӧ�ķ���ʽ����NaOH���������ϡH2SO4������ϡH2SO4����������������
�����ϡH2SO4����������������
����⣺��1����ͼʾ��֪����a��ʱ��Һ��pHС��7��˵�������NaOH��ȫ��Ӧ����ʣ������ᣬ���ԣ���Һ�к��е�����Ϊ��Na+��H+��SO42��
��2����ͼʾ��֪������Һ��pH����7�����ĵ�NaOH��Һ������Ϊ16g�����ʵ�����Ϊ��16g��5%=0.8g��
��3��10gϡ������Ʒ����H2SO4������Ϊx
2NaOH+H2SO4�TNa2SO4+2H2O
80 98
0.8g x
=
��ã�x=0.98g
ϡH2SO4��������������Ϊ��
��100%=9.8%
�ʴ�Ϊ����1��Na+��H+��SO42-����2��0.8����3��ϡH2SO4����������������9.8%
��2����ͼʾ��֪������Һ��pH����7�����ĵ�NaOH��Һ������Ϊ16g�����ʵ�����Ϊ��16g��5%=0.8g��
��3��10gϡ������Ʒ����H2SO4������Ϊx
2NaOH+H2SO4�TNa2SO4+2H2O
80 98
0.8g x
| 98 |
| 80 |
| 0.8g |
| x |
ϡH2SO4��������������Ϊ��
| 0.98g |
| 10g |
�ʴ�Ϊ����1��Na+��H+��SO42-����2��0.8����3��ϡH2SO4����������������9.8%
������������һ�����ͼ��ļ����⣬����ʱ�ҵ�PH=7��ǡ�÷�Ӧ�㣬�����û�ѧ����ʽ�ļ�����н���ǽ����ͻ�ƿڣ�

��ϰ��ϵ�д�
�����Ŀ
 ��
��