��Ŀ����
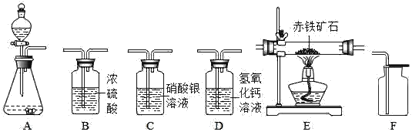
����Ŀ��ij��ѧС������ʦ��ָ��������ͼ��ʾװ��̽��ˮ����ɡ�

��ʵ���¼����ͨ��Դ�������ಣ�����ڹ۲쵽��������____________________________�����Դ���������������ӵIJ��������ռ��������������ԼΪ________��������ͼ�������ڱ�dz���Դ���������� ______���á�+���͡�������ʾ����
���������ϣ�ˮͨ��ֽ����ɵ����������������������
����������裩�ף�A�����ռ����������������B�����ռ����������������ң�A�����ռ����������������B�����ռ���������������
��ʵ��̽�����ף���ȼ�ŵ�ľ�������������壻�ң��ô����ǵ�ľ�������������壻���ڼ���A������ʱ�۲쵽������_________________������B������ʱ�۲쵽________��
��ʵ����ۣ�ˮ����__________________________________������ͬѧ��Ϊʵ�������ײ�ͬ������Ϊ�ڼ���__________�ܣ��A����B��������ʱ���ܻ�û�й۲쵽Ԥ�ڵ�ʵ������
�����������ۣ���ʵ�������ͬѧ�Ƿ������м��©��C��Һ������������ ���������ϣ���֪����������ܶȶ���ˮС���ҷֽ�ˮ�������������ɵ������������������ͣ�����������������ϲ�������Ѿ����յ��������������������ʣ�����C��Һ��������ԭ��_______________________________��
���𰸡��缫��Χ���������ݣ�������������Һ���½���һ��ʱ��� 1:2 �������ҡ�+�� ��ɫ����ȼ�գ����ȣ���������ɫ���� ��ȼ�ŵģ�ľ��ȼ�ո��� ��Ԫ�غ���Ԫ����� A ��Ϊ�ֽ��ˮ��������������������������Ҷ��ߵ��ܶȶ���ˮС������������ռ��������ڷֽ��ˮ�����������Ϊ����������ˮ��������������ˮ����������������������Ϸ�����ˮ������C�У�C��Һ������
��������
����ˮ��ͨ��������������������������������⣬�������1��2������������ȼ�ԣ��������п�ȼ�ԣ��Լ�������������ˮ������������ˮ��֪ʶ���з�����
[ʵ���¼]ˮ��ͨ��������������������������������⣬�������1��2�����Խ�ͨ��Դ�������ಣ�����ڹ۲쵽�������ǣ��缫��Χ���������ݣ�������������Һ���½������Դ���������������ӵIJ��������ռ��������������ԼΪ1��2���������ҡ�+����
[ʵ��̽��]A�ܲ���������B�ܲ�������������������ȼ�ԣ��������п�ȼ�ԣ����Լ��ڼ���A������ʱ�۲쵽�����ǣ���ɫ����ȼ�գ����ȣ���������ɫ���棻����B������ʱ�۲쵽��ȼ�ŵģ�ľ��ȼ�ո�����
[ʵ�����]��ѧ��Ӧǰ��Ԫ������䣬����ˮ������Ԫ�غ���Ԫ����ɣ�����ͬѧ��Ϊʵ�������ײ�ͬ���ڼ���A������ʱ���ܻ�û�й۲쵽Ԥ�ڵ�ʵ������
[����������]��Ϊ�ֽ��ˮ��������������������������Ҷ��ߵ��ܶȶ���ˮС������������ռ��������ڷֽ��ˮ�����������Ϊ����������ˮ��������������ˮ����������������������Ϸ�����ˮ������C�У�C��Һ��������

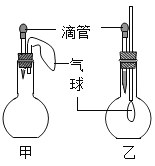
����Ŀ����ͼ��ʾ�ļס�������װ���У���ͷ�ι�������ij��Һ�壬ƽ����ƿ�г��루����룩��һ�����ʣ���ѹ��ͷ�ιܼ���Һ�壬һ��ʱ�����װ���е����������ʹ���Һ����������������Ӱ�죩����ιܺ���ƿ�������Լ�������
�� | �� | |
A | ϡ�����ͭƬ | ˮ�� CO2 |
B | ˫��ˮ��MnO2 | NaOH��Һ��CO2 |
C | Na2CO3��Һ��ϡ���� | NaOH��Һ��SO2 |
D | H2O��NH3 | ��������Һ��HCl |
A.AB.BC.CD.D
����Ŀ����ش���ͼ��Ԫ�����ڱ��е�һ���֣���ش���������.
��һ���� |
|
| ||||||
�ڶ����� |
|
|
| �� |
|
|
|
|
�������� |
|
|
|
|
|
| �� |
|
��1�����У�����ʾԪ�ص�������_______������ʾԪ����ɵĵ��ʵĻ�ѧʽ_______��
��2��![]() ��Ne�ĺ�������Ų���ͬ����X��Ԫ�ط���Ϊ_______��������Ԫ�����ڱ��е�_______���ڣ�������Ԫ����ɵĻ�����Ļ�ѧʽΪ_______.
��Ne�ĺ�������Ų���ͬ����X��Ԫ�ط���Ϊ_______��������Ԫ�����ڱ��е�_______���ڣ�������Ԫ����ɵĻ�����Ļ�ѧʽΪ_______.