��Ŀ����
ζ���dz��õĵ�ζƷ��������ζ���������е���Ҫ�ɷ֡��Ȱ����ơ�����ѧʽ��C5H8NO4Na��������ˮ����AgNO3����Ӧ�����������NaCl�������ɷֲ����ǣ�����ش��������⣺��1��ζ�������ٺ���______��Ԫ�أ������֣���
��2��Ϊ�ⶨNaCl��ζ���е�������������������ʵ�飺
���������Ƶ�50g��Һ�м��������AgNO3��Һ��ַ�ӦNaCl+AgNO3=AgCl��+NaNO3����������Ƿ���ȫ�ķ�����______��
�ڹ��˺���X���������AgCl���壮����X��______��Ŀ����______��
��3����ˮɹ���Ǹ����Ȼ��Ƶ��ܽ�����¶ȱ仯______�����С���������ʣ���ҵ���õ���Ȼ�����Һʱ����һ�ֿ�ȼ�����嵥�ʣ�����______��
���𰸡���������1�������������ʿ����жϳ�ζ���е�Ԫ������
��2���ټ����Ƿ������ȫʵ���Ͼ��Ǽ����Ƿ��������ӣ����Կ�����Ӧ�����Һ�м�����������Ȼ������жϣ�
���ڹ���֮��õ��Ĺ����ϸ�������Һ�����������ˮ�ֵ���������ʹ�������������ӣ�
��3�������Ȼ��Ƶ��ܽ�����¶ȱ仯Ӱ�첻������ֻ�ܹ�ͨ�������ķ������õ��Ȼ��ƣ������Ȼ�����Һ�ijɷֽ������������������жϣ�
����⣺��1��ζ���к��йȰ����ƺ��Ȼ��ƣ��ӻ�ѧʽ�Ͽ�����������Ԫ�أ��ʱ����Ϊ��6��
��2���ټ����Ƿ������ȫʵ���Ͼ��Ǽ����Ƿ��������ӣ����Կ�����Ӧ�����Һ�м��������������û�г�����ȫ����г����������ʱ����Ϊ��ȡ������Ӧ�����Һ���μ�AgNO3��Һ���������ɣ��������ȫ��
�ڹ��˳��ij�������ḽ������Һ��������ʱ������ˮ�ֵ�������ʹ��Һ�еĹ��帽���ڳ������棬��Ӱ�����������������Ҫ�ѹ���������Һ��ϴ�ɾ����ʱ����Ϊ��ϴ�ӣ���ȥ����������Һ��
��3���Ȼ��Ƶ��ܽ�����¶ȱ仯Ӱ�첻������Ҫ����Һ�еõ��Ȼ��ƹ��峣�õķ���������ˮ�����������õ����壬�����Ȼ�����Һ����ɳɷֿ��Եó��ڵ���ʱ��õ��Ŀ�ȼ������Ϊ��������ӦΪ2NaCl+2H2O H2��+Cl2��+2NaOH���ʱ����Ϊ��С��H2������
H2��+Cl2��+2NaOH���ʱ����Ϊ��С��H2������
�ʱ����Ϊ����1��6
��2����ȡ������Ӧ�����Һ���μ�AgNO3��Һ���������ɣ��������ȫ����ϴ�ӣ���ȥ����������Һ��
��3����H2������
�������ܹ��������ʵĻ�ѧʽ���ж����ʵ����Ԫ�أ��������������ӵļ��鷽�����ܹ����������غ㶨�ɵ�ʵ�����жϷ�Ӧ��������ʣ�
��2���ټ����Ƿ������ȫʵ���Ͼ��Ǽ����Ƿ��������ӣ����Կ�����Ӧ�����Һ�м�����������Ȼ������жϣ�
���ڹ���֮��õ��Ĺ����ϸ�������Һ�����������ˮ�ֵ���������ʹ�������������ӣ�
��3�������Ȼ��Ƶ��ܽ�����¶ȱ仯Ӱ�첻������ֻ�ܹ�ͨ�������ķ������õ��Ȼ��ƣ������Ȼ�����Һ�ijɷֽ������������������жϣ�
����⣺��1��ζ���к��йȰ����ƺ��Ȼ��ƣ��ӻ�ѧʽ�Ͽ�����������Ԫ�أ��ʱ����Ϊ��6��
��2���ټ����Ƿ������ȫʵ���Ͼ��Ǽ����Ƿ��������ӣ����Կ�����Ӧ�����Һ�м��������������û�г�����ȫ����г����������ʱ����Ϊ��ȡ������Ӧ�����Һ���μ�AgNO3��Һ���������ɣ��������ȫ��
�ڹ��˳��ij�������ḽ������Һ��������ʱ������ˮ�ֵ�������ʹ��Һ�еĹ��帽���ڳ������棬��Ӱ�����������������Ҫ�ѹ���������Һ��ϴ�ɾ����ʱ����Ϊ��ϴ�ӣ���ȥ����������Һ��
��3���Ȼ��Ƶ��ܽ�����¶ȱ仯Ӱ�첻������Ҫ����Һ�еõ��Ȼ��ƹ��峣�õķ���������ˮ�����������õ����壬�����Ȼ�����Һ����ɳɷֿ��Եó��ڵ���ʱ��õ��Ŀ�ȼ������Ϊ��������ӦΪ2NaCl+2H2O
 H2��+Cl2��+2NaOH���ʱ����Ϊ��С��H2������
H2��+Cl2��+2NaOH���ʱ����Ϊ��С��H2�������ʱ����Ϊ����1��6
��2����ȡ������Ӧ�����Һ���μ�AgNO3��Һ���������ɣ��������ȫ����ϴ�ӣ���ȥ����������Һ��
��3����H2������
�������ܹ��������ʵĻ�ѧʽ���ж����ʵ����Ԫ�أ��������������ӵļ��鷽�����ܹ����������غ㶨�ɵ�ʵ�����жϷ�Ӧ��������ʣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
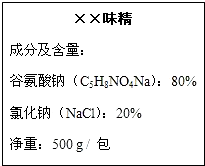 ζ���dz��õĵ�ζƷ��������ζ���������е���Ҫ�ɷ֡��Ȱ����ơ�����ѧʽ��C5H8NO4Na����ijƷ��ζ����װ�ϱ�ע������ͼ��ʾ����ش�
ζ���dz��õĵ�ζƷ��������ζ���������е���Ҫ�ɷ֡��Ȱ����ơ�����ѧʽ��C5H8NO4Na����ijƷ��ζ����װ�ϱ�ע������ͼ��ʾ����ش�