��Ŀ����
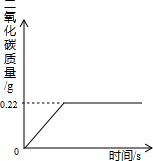 �ڡ������Ƽ���Ĺ��������У����һ�����ü���NaHCO3�ķ�������ȡ����ģ�ij���������ƵõIJ�ƷNa2CO3��������NaHCO3��Ϊ�˲ⶨ��Ʒ��Na2CO3������������ȡ100g�������ȣ�2NaHCO3=Na2CO3+H2O+CO2����Na2CO3���Ȳ��ֽ⣩����Ӧ����������CO2����������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ��
�ڡ������Ƽ���Ĺ��������У����һ�����ü���NaHCO3�ķ�������ȡ����ģ�ij���������ƵõIJ�ƷNa2CO3��������NaHCO3��Ϊ�˲ⶨ��Ʒ��Na2CO3������������ȡ100g�������ȣ�2NaHCO3=Na2CO3+H2O+CO2����Na2CO3���Ȳ��ֽ⣩����Ӧ����������CO2����������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ��
�����������⣺
��1����Ӧ����������CO2������________g��
��2��100g�������NaHCO3��������
��3���������Na2CO3������������
�⣺��1����ͼ���֪�����ɶ�����̼������Ϊ0.22g
��2����������NaHCO3������Ϊx
2NaHCO3 Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2��
168 44
x 0.22g
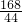 =
=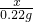
x=0.84g
��3���������Na2CO3������������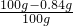 ��100%=99.16%
��100%=99.16%
�𣺣�2��100g�������NaHCO3������Ϊ0.84g
��3���������Na2CO3����������Ϊ99.16%
����������ͼ���֪��Ӧ�����Ķ�����̼������Ϊ0.22�ˣ���Ҫ���NaHCO3��������Na2CO3��������������ؼ�����Ҫ֪��ֻ��NaHCO3����ʱ����ֶ�����̼���壬�������ǾͿ��Ը��ݶ�����̼������ͨ������ʽ���Ƶ���NaHCO3���������Ӷ����������Na2CO3������������
�����������Ĺؼ���Ҫѧ���ͼ��Ū��ͼ���۵������ĺ��壬����ͼ�Ż����������⣮
��2����������NaHCO3������Ϊx
2NaHCO3
 Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2��168 44
x 0.22g
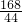 =
=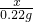
x=0.84g
��3���������Na2CO3������������
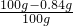 ��100%=99.16%
��100%=99.16%�𣺣�2��100g�������NaHCO3������Ϊ0.84g
��3���������Na2CO3����������Ϊ99.16%
����������ͼ���֪��Ӧ�����Ķ�����̼������Ϊ0.22�ˣ���Ҫ���NaHCO3��������Na2CO3��������������ؼ�����Ҫ֪��ֻ��NaHCO3����ʱ����ֶ�����̼���壬�������ǾͿ��Ը��ݶ�����̼������ͨ������ʽ���Ƶ���NaHCO3���������Ӷ����������Na2CO3������������
�����������Ĺؼ���Ҫѧ���ͼ��Ū��ͼ���۵������ĺ��壬����ͼ�Ż����������⣮

��ϰ��ϵ�д�
 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�
�����Ŀ
ijѧУ��ѧ�С���ͬѧ��ȡ�ս��������ˮ������pH�ƣ���pH��������ÿ�������Ӳ�һ��pH�������±����ݣ�������ν�ˮ�����˵����ȷ����
| �ⶨʱ�� | 5��05 | 5��10 | 5��15 | 5��20 | 5��25 | 5��30 | 5��35 |
| pH | 4.95 | 4.94 | 4.94 | 4.88 | 4.86 | 4.85 | 4.85 |
- A.��ˮ���嶼ƫ����
- B.�ⶨ���ڼ��ڣ���ˮ�ļ�����ʱ������ӱ�ĸ�ǿ
- C.��ˮ����Ի������Σ��
- D.��ˮ���ʺ�ũ���������
Ϊ�˲ⶨijͭп�Ͻ���ͭ������������ȡ�Ͻ���Ʒ��ϡ���ᷴӦ������ʵ����й��������±���
| ʵ���� | ����ϸ���Ʒ������/g | ��ȡϡ���������/g | ��������������/g |
| 1 | 10.0 | 40.0 | 0.1 |
| 2 | 10.0 | 50.0 | 0.1 |
| 3 | 20.0 | 36.5 | 0.1 |
��2�������ͭп�Ͻ���ͭ������������________��

 ��Si��
��Si�� ����������һ�������մ�ԭ�ϵ���Ҫ�ɷ֣��ܳ��ܸ��£�����������ҵ������ҵ�ȣ���д��������Ļ�ѧʽ________��
����������һ�������մ�ԭ�ϵ���Ҫ�ɷ֣��ܳ��ܸ��£�����������ҵ������ҵ�ȣ���д��������Ļ�ѧʽ________�� A��B���ֹ�����ܽ��������ͼ��ʾ��ij�ձ���ʢ�к�A��B�������ʵ�60��ʱ�ı�����Һ���ұ�����������A��B���壬�����¶ȵĽ��ͣ���
A��B���ֹ�����ܽ��������ͼ��ʾ��ij�ձ���ʢ�к�A��B�������ʵ�60��ʱ�ı�����Һ���ұ�����������A��B���壬�����¶ȵĽ��ͣ���