��Ŀ����
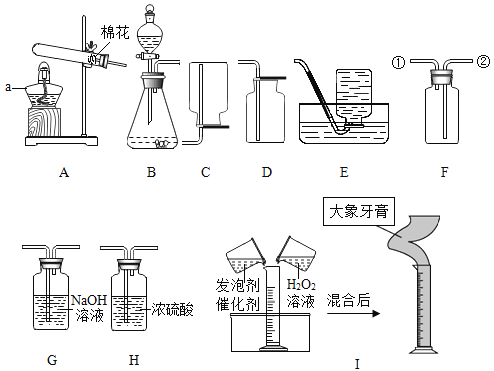
����Ŀ����10�֣�Ϊ̽������X����ɣ�ij��ȤС�����ͼ12��ʾʵ�飨�̶�װ��δ��������
���������ߡ�
��1�����������£�����X��������ͭ��Ӧ����ͭ��ˮ�͵�����
��2����ʯ��Ϊ�����������ƺ������ƵĻ�����������X��Ӧ��
��ˮ�Ȼ��ƿ���������X��
��3����ʵ�������£������ܶ�Ϊ1.15g��L-1��װ���ڿ����е�ˮ�������̼��������ݲⶨ��Ӱ��ɺ��Բ��ơ�
��ʵ�鲽�衿
��1�����������������װ�������ԡ�
��2��ȡһ��������ͭ��ȷ�Ƶ�������Ϊ2.40g��
��3����ͼ12��ʾװ��ҩƷ����������¼���������������1����
��4������A��B��Cװ�ã� ������X����ͨ��һ��ʱ���������Dװ�ã�������ʼ���ȡ���Aװ��������ͭ��Ӧ��ȫ��ֹͣ���ȣ�����ͨ������ X����������ȴ���ٴβ�������¼���������������1����
��ʵ��װ�á�

��������ݡ�

���ش����⡿
��1��Bװ������������ˮ��ԭ��Ϊ ���û�ѧ����ʽ��ʾ����
��2��ʵ������У����۲쵽 ��˵������ͭ�ѷ�Ӧ��ȫ��ֹͣ���ȣ�����ͨ������X����������ȴ��Ŀ���� ��
��3������ʵ����������ݣ�����ˮ������Ϊ g������ͭ����Ԫ�ص�����Ϊ g��
�ɴ���֪������X��һ�� ����С��� ��û�С�����Ԫ�ء�
��4��ͨ�������Ƶ�������X�Ļ�ѧʽ��
���𰸡� ��1��CaO+H2O==Ca(OH)2 ��2����������Һ�治���½� ��ֹ���ȵ�ͭ������
��3��0.54 0. 48 û�� ��4��NH3
��������
�����������1����������ˮ����Ϊ��������ˮ��Ӧ�����������ƣ���Ӧ�Ļ�ѧ����ʽΪ��CaO+H2O==Ca(OH)2
��2���������������е����ݿ�֪��������X��������ͭ�ڼ��ȵ�������Ӧ����ͭ��ˮ�����͵����������е�����ͨ��Bװ��ʱ��ˮ���������գ���ͨ��Cװ��ʱ�������X���屻���գ������Dװ���ڵ�����Ϊ��Ӧ���ɵĵ��������ŵ�������Dװ�ã���������е��������࣬��ѹ���۲쵽Һ���½������Ե��۲쵽��������Һ�治���½�ʱ��˵���������ɵ���������˵������ͭ�ѷ�Ӧ��ȫ��������������X�Ƿ�ֹ���ȵ�ͭ���������ʷ�Ӧ����������
��3����������ķ�������ϱ�1��֪��װ��B���������������ɵ�ˮ������װ��B����������ֵ��Ϊ����ˮ����������102.54g��102.00g=0.54g���ڲ�������ԭΪ����ͭ����Ӧ��������ɷ�Ϊͭ���ʣ� ��������������ֵ��Ϊ����ͭ����Ԫ�ص���������Ϊ52. 40g��51.92g= 0.48g��Ҳ�ɸ�������ͭ�����������Ԫ�ص�����������һ���ɸ���װ��B�����ˮ�����������ˮ�Ļ�ѧʽ��H2O���������������Ԫ�ص�����Ϊ��0.54g����![]() ��=0.48g�������������Ԫ�ص�������ͬ����˵��������X��û����Ԫ�ء�
��=0.48g�������������Ԫ�ص�������ͬ����˵��������X��û����Ԫ�ء�
��4����������������֪��������ֻ����Ԫ�غ���Ԫ�أ��ҿɸ��ݵ���������������е�Ԫ�ص��������ɸ�������ˮ���������������Ԫ�ص�������Ȼ��ɸ��ݵ�Ԫ������Ԫ�ص���������������е���Ԫ�ص�ԭ�Ӹ����ȣ�����ȷ���仯ѧʽ���������������£�
�⣺�������Ļ�ѧʽΪNxHy�����������֪��
14x��1y=��0.2435L��1.15g/L������0.54g��0.48g��
x��y=1��3
���Ը�����Ļ�ѧʽΪNH3

 ���������ν�ϵ�д�
���������ν�ϵ�д�