��Ŀ����
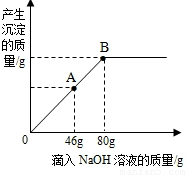
 ��ʢ��102g����������þ��Һ���ձ��У���ε���������������Ϊ10%��NaOH��Һ������������������������NaOH��Һ��������ϵ������ͼ��ʾ��
��ʢ��102g����������þ��Һ���ձ��У���ε���������������Ϊ10%��NaOH��Һ������������������������NaOH��Һ��������ϵ������ͼ��ʾ�����������ش��������⣺
��Ӧ����ʽΪ��MgSO4+2NaOH�TNa2SO4+Mg��OH��2��
��1��������NaOH��Һ��ͼ��A��ʱ���ձ�����Һ�ﺬ�е������ǣ�д��ѧʽ��
��2��������10%��NaOH��Һ80gʱ����B�㣩����ͨ�����㣬���ʱ������Һ�����ʵ�����������
���������ݡ���������������������NaOH��Һ��������ϵ���ߡ��������ŵμ�NaOH��Һ��Ӧ�Ľ����������ǡ����ȫ��Ӧʱ��B�㣩��������ҺΪ��������Һ�����ݻ�ѧ����ʽ����������������Ƶ����������������غ㶨�ɣ������������Һ�����������ɽ�����⣮
����⣺��1���������߿�֪��������46gNaOH��Һʱ������þû����ȫ��Ӧ����ˣ���ʱ��Һ�к���δ��ȫ��Ӧ������þ���Ѿ���Ӧ���ɵ������ƣ�
�ʴ�Ϊ��MgSO4��Na2SO4��
��2���裺����Na2SO4������Ϊx��Mg��OH��2����Ϊy
MgSO4+2NaOH�TNa2SO4+Mg��OH��2��
80 142 58
80g��10% x y
=
��֮�� x=14.2g
=
��֮�� y=5.8g
������Һ��Na2SO4���ʵ���������=
��100%=8.1%
�𣺴�ʱ������Һ�����ʵ���������Ϊ8.1%��
�ʴ�Ϊ��MgSO4��Na2SO4��
��2���裺����Na2SO4������Ϊx��Mg��OH��2����Ϊy
MgSO4+2NaOH�TNa2SO4+Mg��OH��2��
80 142 58
80g��10% x y
| 80 |
| 80g��10% |
| 142 |
| x |
| 80 |
| 80g��10% |
| 58 |
| y |
������Һ��Na2SO4���ʵ���������=
| 14.2g |
| 102g+80g-5.8g |
�𣺴�ʱ������Һ�����ʵ���������Ϊ8.1%��
���������������غ㶨�ɣ���Ӧ��������Һ������=����þ��Һ������+�����NaOH��Һ����-����������þ����������

��ϰ��ϵ�д�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д� ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д�
�����Ŀ
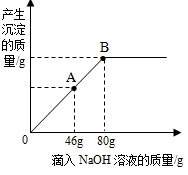
 ��ʢ��102g����������þ��Һ���ձ��У���ε���������������Ϊ10%��NaOH��Һ������������������������NaOH��Һ��������ϵ������ͼ��ʾ��
��ʢ��102g����������þ��Һ���ձ��У���ε���������������Ϊ10%��NaOH��Һ������������������������NaOH��Һ��������ϵ������ͼ��ʾ��