��Ŀ����
��7�֣� ��������ʵ�鳣��װ�ã��ش��й����⡣

A B C D E
(1)д��ͼ�б�����ĸ���������ƣ�a_________________��
(2)���ø��������ȡ������Ӧѡ�õķ���װ����__________����װ�õ���ţ�����Ӧ�Ļ�ѧ����ʽΪ ��
(3)��Ҫ��ȡ������̼����Bװ����װ��ʯ��ʯ����ôa��Ӧ����___________����Ҫ��ø���Ķ�����̼����Ӧѡ��Dװ�ã����ڸ�װ����ʢ�� ����д�Լ����ƣ��������Eװ���ռ������壬�������_________�˽��루�b����c������
(4)ʵ�������ÿ�״�����Һ�����������ȡ���壬�ɽ�Bװ�øĽ�ΪCװ�ã�����������ſ�״���壩����Ľ�����ŵ��� ��
��1������©��
��2��A ��
2 KMnO4 K2MnO4 + MnO2
+ O2��
K2MnO4 + MnO2
+ O2��
��3��ϡ���� Ũ���� b
��4��������ʱ���Ʒ�Ӧ�ķ���
��������
�����������1����Ϥ�����������˽����ƣ�ͼ��a�dz���©����
��2����ȡ�����װ�÷�Ϊ����װ�ú��ռ�װ�ã�����װ����Ҫ���Ƿ�Ӧ���״̬�ͷ�Ӧ�������ռ�װ����Ҫ���������ˮ���Ժ��ܶ�������Ĵ�С�����ڸ�������ǹ��壬���ø��������ȡ����ʱ��Ҫ���ȣ�����Ӧ��ѡ��Aװ������Ϊ����װ�ã����ڸ���������ȷֽ���������ء��������̺��������ʸ÷�Ӧ�Ļ�ѧ����ʽΪ2KMnO4 K2MnO4+MnO2+O2����
K2MnO4+MnO2+O2����
��3������©�������þ��Ƿ�������ƿ�м���ϡ����ģ�������̼�����������壬����Ӧ��ѡ��Ũ�������������Dװ����Ӧ�ü���Ũ������ڶ�����̼���ܶȱȿ����Ĵ���������Eװ�����ռ�������̼ʱ��Ҫ�������ſ������ռ�����Ӧ�ô�b�ܽ��롣
��4��Cװ����һ�����������շ�������װ�ã��ʿ��Բ������շ��������ŵ��������װ��C�м����˶���壬����ͨ�����ɼ������Ʒ�Ӧ������Ҫ��ʱ����ʱ��ʼ����ֹʵ�顣
���㣺��������ȡװ�ã�������̼��ʵ�����Ʒ�
��������������ʵ��������ȡ�����������װ�ã��ܹ����ݷ�Ӧ���״̬�ͷ�Ӧ������ѡ����ʵķ���װ�ã��ܹ�����������ܶȺ�ˮ������ѡ����ʵ��ռ��������ǽ������Ĺؼ���

 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�

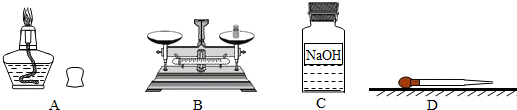

 ��������ʵ�鳣��װ�ã��ش��й����⣮
��������ʵ�鳣��װ�ã��ش��й����⣮